

Higher and foundation tier
Aluminium is an attractive, corrosion resistant lightweight shiny metal. It has many desirable properties and uses, a few of which are illustrated below:
Aluminium and its alloys are low density, corrosion resistant alloys with an excellent strength to weight ratio and excellent fatigue resistance which makes them ideal for use in the aviation and car industries. Aluminium alloys are the primary material used to make alloy wheels for cars and the body panels in many sports and electric cars. Compared to steel, aluminium alloys are significantly lighter. This translates to better fuel efficiency, improved handling.
 |
 |
Copper metal is used as an electrical conductor in the electrical wires in our home and while aluminium is not as good an electrical conductor as copper it is significantly lighter. The low density of aluminium is a major factor in favour of its use in overhead power lines and cables, particularly for long distance transmission cables on pylons where the electrical cables can run for many miles. Aluminium is also much cheaper than copper, making it a more economical choice for power lines. Even though aluminium conducts electricity less efficiently than copper (around 61% as conductive), its weight advantage makes up for this. Thicker aluminium wires can be used to achieve the same conductivity as thinner copper wires, but they'll still weigh less.
Aluminium is a good thermal conductor and transfers heat well compared to many other materials. This makes it useful in applications where heat dissipation is important, for example in heat sinks in many electronics devices or in pots and pans used for cooking.
 |
 |
 |
Aluminium is a corrosion resistant metal due to the fact that it forms a thin, invisible and tough layer of aluminium oxide on its surface when it comes into contact with air or oxygen. This layer thin layer of aluminium oxide acts as a shield, protecting the aluminium metal underneath from further oxidation. This naturally resistant to corrosion makes aluminium suitable for use in harsh weather conditions. It can withstand rain, wind, and extreme temperatures without deteriorating. This corrosion resistance along with its low density makes it an ideal material to use as cladding on buildings, as shown in the image below.
However aluminium is a fairly soft metal and is prone to dents and scratches, especially from hail, strong winds, or flying debris. These imperfections can detract from the building's appearance and may require panel replacements which could be an expensive business. Recently there have been some fires in high-rise building which spread quickly, some types of aluminium cladding, particularly Aluminium Composite Panels (ACPs) with flammable cores have raised fire safety concerns. Strict regulations and fire safety tests are crucial when choosing ACPs to ensure they meet building codes and reduce the risks associated with fires in high-rise buildings and offices.
Aluminium is also an attractive metal which can be painted, anodised or powdered coated and it can also be formed into various shapes and panels, allowing for creative modern looking architectural designs in buildings and offices.

Aluminium is a fairly strong, lightweight and attractive metal which can offer good impact protection makes it an ideal material for items such as mobile phone cases. Being a lightweight material means it won't add significant bulk to your phone, making it comfortable to carry around. Since aluminium is a good thermal conductor this can help draw heat away from your phone, particularly beneficial during activities that generate heat, such as gaming or video conferencing. This can help prevent overheating and improve the performance of the mobile phone. Aluminium can be machined and finished in various ways, allowing for sleek and stylish phone case designs. Aluminium can also be anodized for a variety of colours or left with a natural metallic finish and finally unlike some other metals, aluminium doesn't typically interfere with mobile cell phone signals to a significant degree. This ensures you maintain good connectivity.
Perhaps the best known use for aluminium is as cooking foil. As mentioned above aluminium metal conducts heat very efficiently this ensures that any foods wrapped in it during cooking cook evenly. Aluminium foil acts as a barrier to light, air, and moisture. This is crucial for food storage as it helps prevent spoilage caused by light or air exposure and locks in moisture to keep food from drying out, ideal to ensure your sandwiches don't dry out!
 |
 |
Aluminium is the third most abundant element in the Earth crust; its main ore is called
bauxite. Bauxite
contains a high proportion of aluminium compounds, chiefly aluminium oxide (Al2O3). The
aluminium oxide
contains positively charged aluminium ions (Al3+) and negatively charged oxide ions (O2-) ions. It is named after a village
in southern France called Les Baux, where it was first discovered in 1821.
Despite the fact that aluminium
is the third most common element on Earth in the late 19th century it was more valuable than silver or gold and
it was considered a rare metal. The reason for this strange fact is that before the discovery of electrolysis
aluminium was extracted by carrying
out a displacement reaction using sodium or potassium on aluminium compounds, this made it a very expensive
metal
indeed.
However with the discovery of the electrolytic process the cost of aluminium fell dramatically. However the
extraction of aluminium by electrolysis was not as straightforward as might have been expected at first. In
order to extract a metal from its ore by electrolysis
the metal ore needs to be molten (melted to form a liquid)
or dissolved to form a solution. However bauxite is not soluble in many solvents and it also has a very high
melting point, in excess of 20000C. This made the extraction of aluminium from its
ore difficult and expensive.
The solution to the problem was found by two scientists working independently from each other, Charles Hall an
American scientist and Paul Héroult a French scientist found that aluminium oxide would dissolve in
cryolite (Na3AlF6),
a rare mineral found in Greenland.
If cryolite is heated to around 9500C then
aluminium oxide will
dissolve in it and
form a solution which conducts electricity. This made it possible to extract aluminium by
electrolysis from its ore
relatively cheaply and following this the cost of aluminium metal plummeted.
Aluminium oxide (Al23+O32-) dissolves in the molten
cryolite inside the
cell where temperatures are around 9500C.
When it dissolves its giant lattice structure is broken down and the aluminium and oxide ions are free to move. This
is exactly the same as what would happen if an ionic compound was dissolved in water, only in this case it is dissolved
in molten cryolite. The image below shows a typical cell used to extract aluminium.

The cell is lined with a layer of graphite; this graphite is made the cathode. This negatively charged cathode will attract the positively charged aluminium ions and reduce them to aluminium atoms:

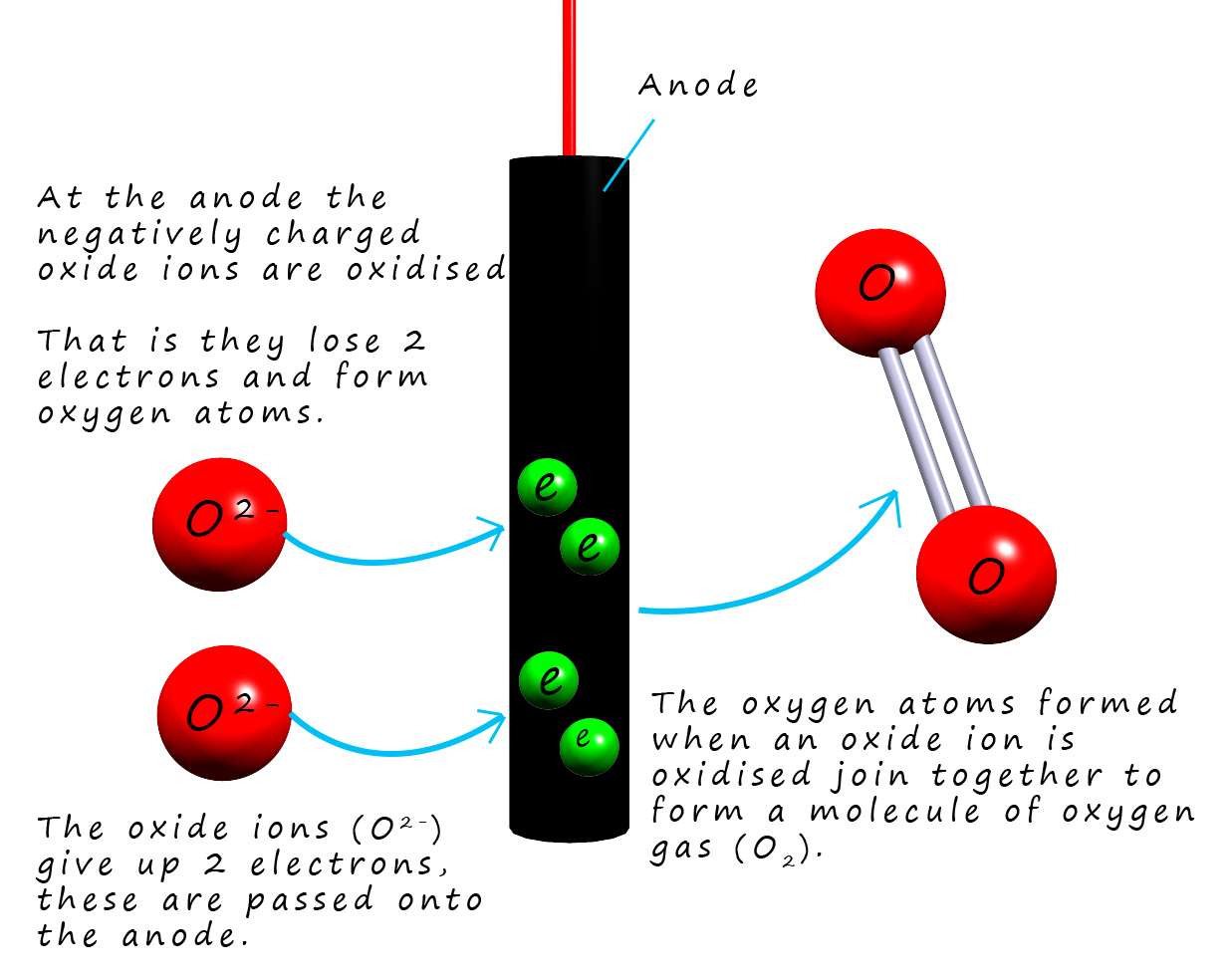 At the positively charged anode the negatively charged
oxide ions (O2-) are attracted and they are oxidised
(lose electrons) to form oxygen gas (O2). The half equation for this oxidation reaction is:
At the positively charged anode the negatively charged
oxide ions (O2-) are attracted and they are oxidised
(lose electrons) to form oxygen gas (O2). The half equation for this oxidation reaction is:To obtain the overall equation for the reaction taking palce in the cell it is simply a matter of combining the two half-equations for tha cathode and anode reactions:
One of the major costs in extracting aluminium is the cost and energy required to continually make new anodes as the old one burn away and turn into carbon dioxide gas. The other major consideration in aluminium extraction is the vast amount of electricity required to extract aluminium from its ore. Huge electrical currents in excess of 150 000 amps are used and so a cheap source of electricity nearby is required. A hydro-electric power station would be ideal!