

Higher and foundation tiers
Electrolysis (electro:electricity and lysis: splitting) as its name suggests is using electricity to break up Ionic compounds into the elements that make them up. Recall that ionic compounds are made up of positively charged metal ions and negatively charged non-metal ions. Metals when they react tend to lose electrons and end up forming ions with a positive charge while non-metals when they react gain electrons and end up forming ions with a negative charge. We can summarise this as:
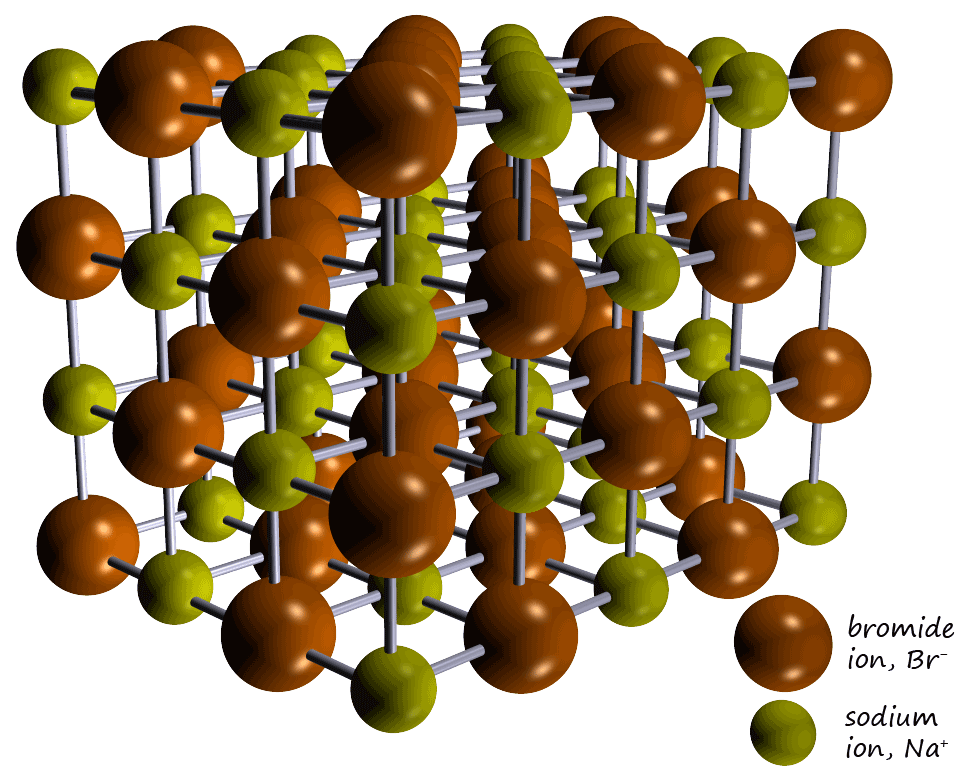 All ionic compounds have a giant lattice structure made up of positively charged metal ions and negatively charged non-metal ions with
strong bonds between the metal and non-metal ions. The ionic lattice for the ionic compound sodium bromide is shown opposite.
It contains positively charged sodium ions (Na+) and negatively charged bromide ions (Br-) which having opposite
charges are
strongly attracted to each other. The ions are
held tightly within the giant ionic lattice structure (shown opposite) and are
NOT free to move. This
however creates a bit of a problem!
All ionic compounds have a giant lattice structure made up of positively charged metal ions and negatively charged non-metal ions with
strong bonds between the metal and non-metal ions. The ionic lattice for the ionic compound sodium bromide is shown opposite.
It contains positively charged sodium ions (Na+) and negatively charged bromide ions (Br-) which having opposite
charges are
strongly attracted to each other. The ions are
held tightly within the giant ionic lattice structure (shown opposite) and are
NOT free to move. This
however creates a bit of a problem!
In order for electrolysis to work electricity
must be able to flow through
the substance and SOLID ionic compound are electrical insulators; they do not conduct
electricity. In order to
conduct an electrical current the ions must be free to move and
this is simply not the case in SOLID ionic compound.
However if the solid ionic compound is melted
or dissolved in water then the giant ionic lattice is broken down and
the ions are free to move; this means that molten ionic compounds (liquids) and
solutions of
ionic compounds will
conduct electricity.
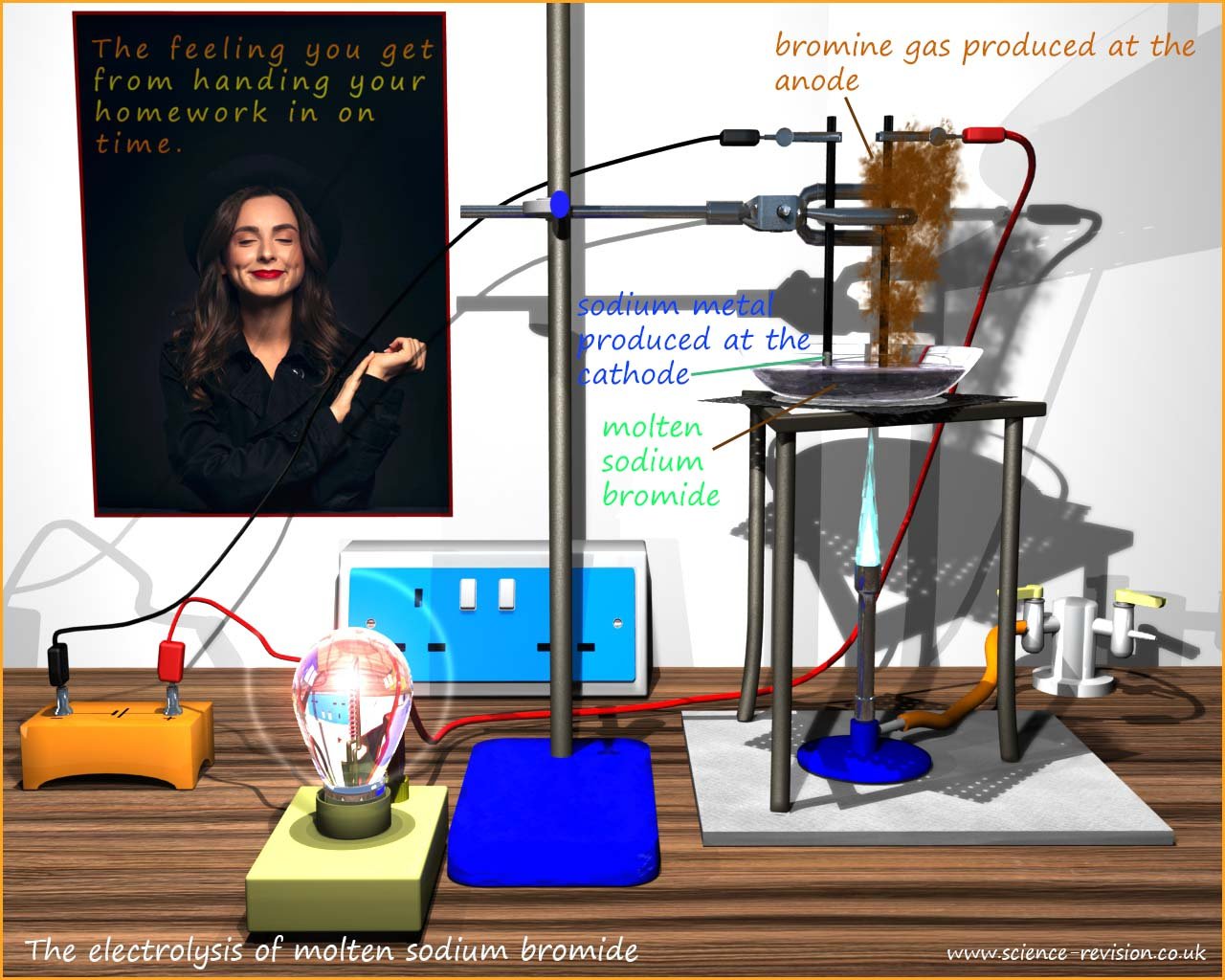
We can easily demonstrate this by placing solid sodium bromide in a crucible with two electrodes connected to a
battery pack (see image below). If a bulb is included in the circuit then it will light up when an electrical current is flowing. To begin with
the bulb is unlit but as the sodium bromide begins to melt and becomes molten the metal and non-metal
ions within the structure are free to move and the
bulb now begins to glow as an electrical current flows.
If you look carefully at the electrodes in the image below you will see that a brown, bleachy smelling gas is
produced at the positive
terminal (the anode) and a silvery grey metallic solid coats the negative electrode (the cathode).
Electrolysis basically turns ions back into atoms. This means forcing
electrons back onto positively charged
metal ions and turning
them back into neutral metal atoms; reducing them. At the same time electrons are pulled off the negatively charged
non-metal ions; oxidising them and turning
them back into non-metal atoms. The apparatus shown in the diagram above can be drawn out in a simple way to show what is happening in
the cell (see image below).
The electrodes in the cell are made of graphite; an inexpensive but
good electrical conductor. Graphite also
plays no part in any of the reactions which take place during electrolysis; that is it is inert and so it will not interfere with the electrolysis experiment. The graphite electrode
connected to the negative terminal of the cell is called the cathode and the electrode connected to the positive
terminal of the cell is called the anode. They are dipped in the electrolyte (a solution which conducts electricity);
in this case the molten sodium bromide.
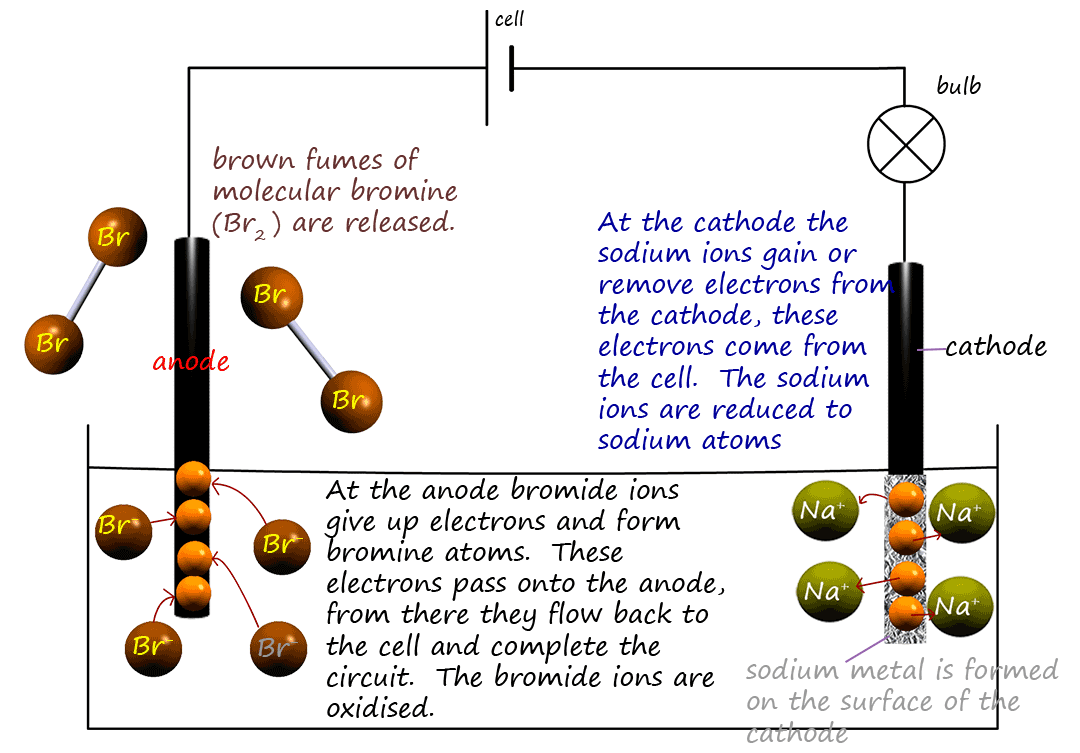 The cell or battery releases electrons from the negative terminal and these electrons travel to the cathode.
However once there the positively charged sodium ions pick them off and
are they reduced according to the equation below:
The cell or battery releases electrons from the negative terminal and these electrons travel to the cathode.
However once there the positively charged sodium ions pick them off and
are they reduced according to the equation below:

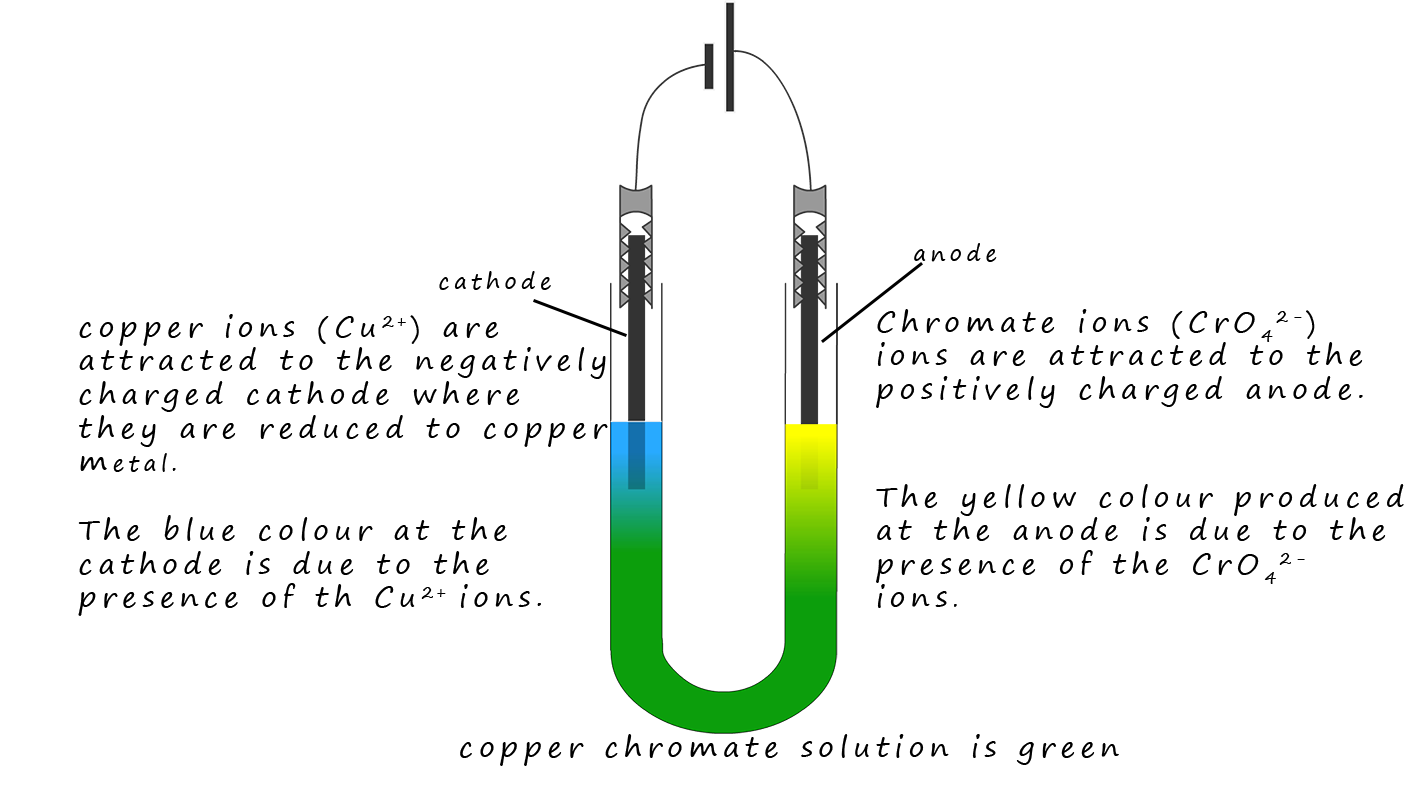 Copper chromate is an ionic compound containing blue copper ions (Cu2+) ions and yellow chromate ions
(CrO42-). These two ions
mix and the resulting solution is green. However when the solution is
electrolysed the positively charged
(Cu2+) ions are attracted to the negatively charged cathode while the
yellow CrO42- ions are attracted to the positively charged anode. This simple
demonstration is a good piece of evidence for the presence of ions in a solution.
Copper chromate is an ionic compound containing blue copper ions (Cu2+) ions and yellow chromate ions
(CrO42-). These two ions
mix and the resulting solution is green. However when the solution is
electrolysed the positively charged
(Cu2+) ions are attracted to the negatively charged cathode while the
yellow CrO42- ions are attracted to the positively charged anode. This simple
demonstration is a good piece of evidence for the presence of ions in a solution.
| molten ionic compound | cathode product | cathode half-equation | anode product | Anode half-equation | observations |
|---|---|---|---|---|---|
| potassium oxide | potassium | K+ + e → K | oxygen | 2O2- → O2 + 4e | bubbling at anode as oxygen released. |
| lithium chloride | lithium | Li+ +e → Li | chlorine | 2Cl- → Cl2 + 2e | green/yellow gas produced at anode. Smells of bleach and bleaches litmus paper white. Silvery metal produced at cathode. |
| copper iodide | copper | Cu2+ +2 e → Cu | oxygen | 2I- → I2 + 2e | violet coloured vapours produced at the anode. Cathode coated in a bronze/brown coloured metal. |