

Higher and foundation tiers
Displacement reactions are a good way to demonstrate the order of reactivity of metals. In a
displacement reaction a
more reactive metal will remove or displace a less reactive metal from its compound or solution.
The reactivity series for metals is shown lower down this page; it will help you work out what is happening in a displacement reaction. You may notice that two non-metals, carbon and hydrogen have
been included in the reactivity series. This is simply because these are two common reagents that are frequently reacted with metal compounds; often to extract metals from their ores.
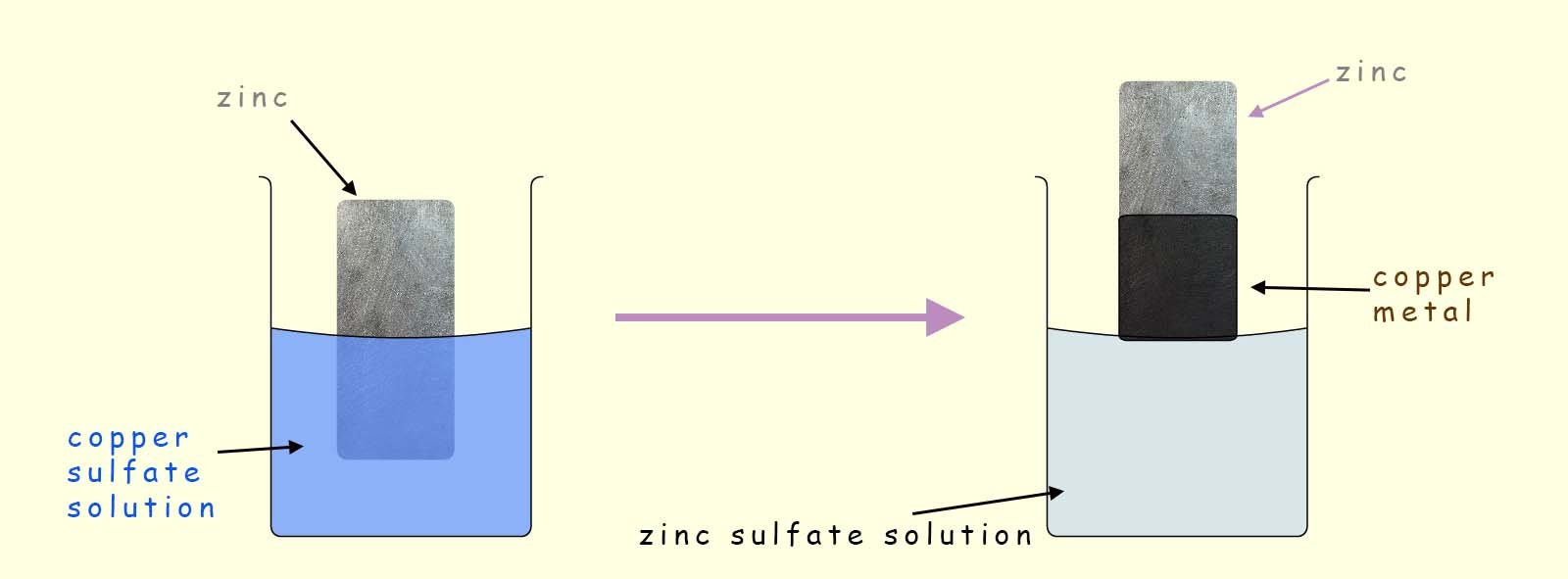
Consider the following displacement reaction where a strip of zinc metal is dipped into a copper sulfate solution for about 30 seconds. When the strip of zinc is removed it is covered in a thin layer of black copper powder (note copper metal is usually a bronze colour but damp copper powder appears black!), this is outlined in the image below:
| potassium |
| sodium |
| lithium |
| calcium |
| magnesium |
| aluminium |
| carbon |
| zinc |
| iron |
| tin |
| lead |
| hydrogen |
| copper |
| silver |
| gold |
| platinum |
In this displacement reaction there are 2 metals present; copper and zinc. From the reactivity series shown opposite you can see that zinc is the more reactive metal of the two. Now in a displacement reaction the more reactive metal displaces or removes the less reactive metal from its compound or solution.
So in this example the zinc metal will displace or remove the copper ions from the copper sulfate solution (Cu2+SO42-). Word and symbolic equations for this displacement reaction are shown below:
An ionic equation is simply the symbolic equation with the charges on the ions shown, these ionic equations are helpful in identifying
what has been reduced and what has been oxidised during a chemical reaction. The ionic equation for this reaction is:
Displacement reactions like the one described above involving solutions are fun and safe to carry out in the lab. However reactions involving solids are much more violent and much more care is needed to ensure they are carried out safely. The diagram below illustrates the reaction between magnesium powder and copper oxide. Here the more reactive magnesium metal displaces or removes the less reactive copper from the solid copper oxide. This reaction is very violent!
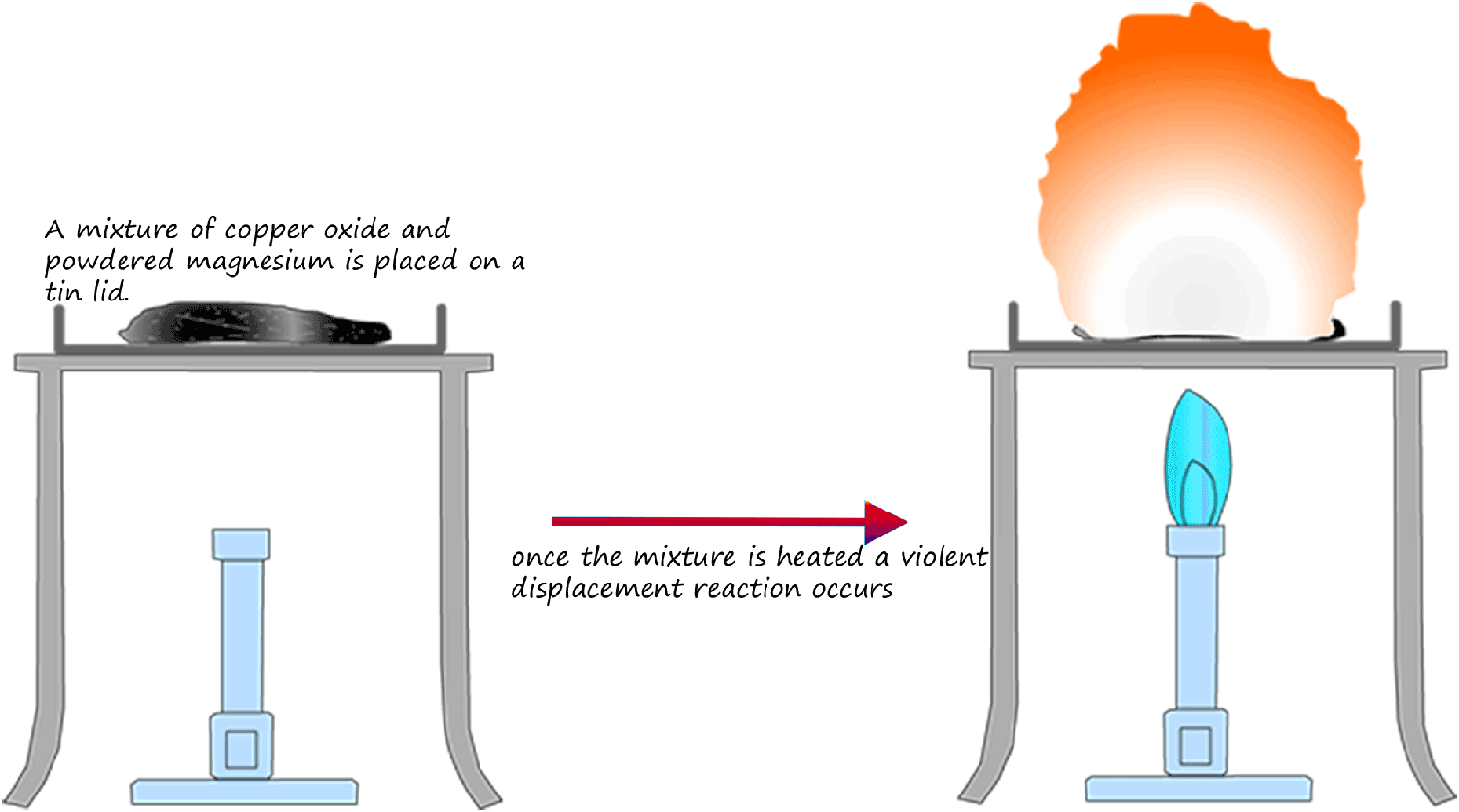
Equations for this displacement reaction are:
The further apart the two metals are in the reactivity series the more violent and explosive the reactions become. If the copper oxide in the above displacement reaction is replaced with silver nitrate then an explosive reaction occurs, this reaction is extremely violent and a large amount of heat and light is released in the resulting explosion. The reaction is more violent since the two metals involved; silver and magnesium are further apart in the reactivity series than copper and magnesium. Once the two reactants are mixed a simple drop of water will cause this explosive reaction to start, there is no need to heat the mixture. The reaction can be summarised as:
In both these examples the more reactive metal is oxidised, it loses electrons and the less reactive metal is reduced, it gains electrons. These reactions are displacement reactions but they are also redox reactions.
Hydrogen, though not a metal reacts in many cases as if it was a metal. In terms of reactivity hydrogen sits just above copper in the reactivity series. This means that it can be used to displace any metal below it in the reactivity series such as copper or silver. The image below shows how copper oxide can be reduced to copper metal using a stream of hydrogen gas from a cylinder.

Equations to show this displacement reaction are shown below:
Review your understanding of metal displacement reactions by completing the activity below. For each example predict if any reaction will occur and try to write word, symbolic and ionic equations for each reaction. Pressing the "reaction occurs" or "no reaction" buttons will provide the equations for any reaction that occurs.
Sort the statements below into either the true or false bins, when you are done press the check answers button.
Drag statements into the bins or click a statement, then click a bin.