
Higher and foundation tiers

Metals are very valuable elements with many desirable properties and uses. You only have to take a look around at the many objects
that are made from metals and alloys to realise how useful
metals are. However very few metals are found as
pure elements in the Earth's crust.
Most metals will react with
whatever elements; such as oxygen and sulfur which are around them to form compounds. These compounds are found in rocks and minerals and it is from these that the metals we depend upon are extracted.
Metal
ores are rocks which contain compounds with a high enough proportion of a metal in them to make it economically viable to extract the metal from them. The images below show two common metal ores, haematite (iron oxide) is a common ore of iron while chalcocite (copper sulphide) is an ore containing copper metal.
If it is economic to extract a
metal from
its ore then it may be mined out of the ground and the metal extracted from them. If the
metal ore
contains an expensive metal such as copper it may be economically viable to extract it even if the amount
of copper present is low. However if the ore contains a small amount of a less valuable
metal such as iron
then it would not be economically viable to extract it.

| potassium |
| sodium |
| lithium |
| calcium |
| magnesium |
| aluminium |
| carbon |
| zinc |
| iron |
| tin |
| lead |
| hydrogen |
| copper |
| silver |
| gold |
| platinum |
Consider the reactions of two highly sought after metals, lithium and iron with oxygen. Lithium an alkali metal is fairly close to the top of the reactivity series and iron which is used to make steel is roughly in the middle of the reactivity series. Equations for the reaction of these two metals with oxygen are shown below:
Lithium is so much more reactive than iron, it releases a lot more energy than iron does when it reacts with oxygen. This means that in order to reverse the above reactions and extract the metals from their compounds then we have to put back more energy to extract lithium than iron, the same amount of energy that was released when the metal reacted will have to be put back in order to extract the metal from its ore.
Reactive metals, that is metals above carbon in the reactivity series are extracted from their ores using a method called electrolysis. This involves passing a large electrical current through the melted or molten ore. Metal ores or "rocks" will have high melting points so melting these ores will require large amounts of energy, also once the ore is melted to extract the metal from it a large amount of expensive electricity will need to be passed through the molten ore. So extracting reactive metals such as aluminium, calcium, sodium, lithium or potassium from their ores will be an energy intensive process and so these metals will be expensive.
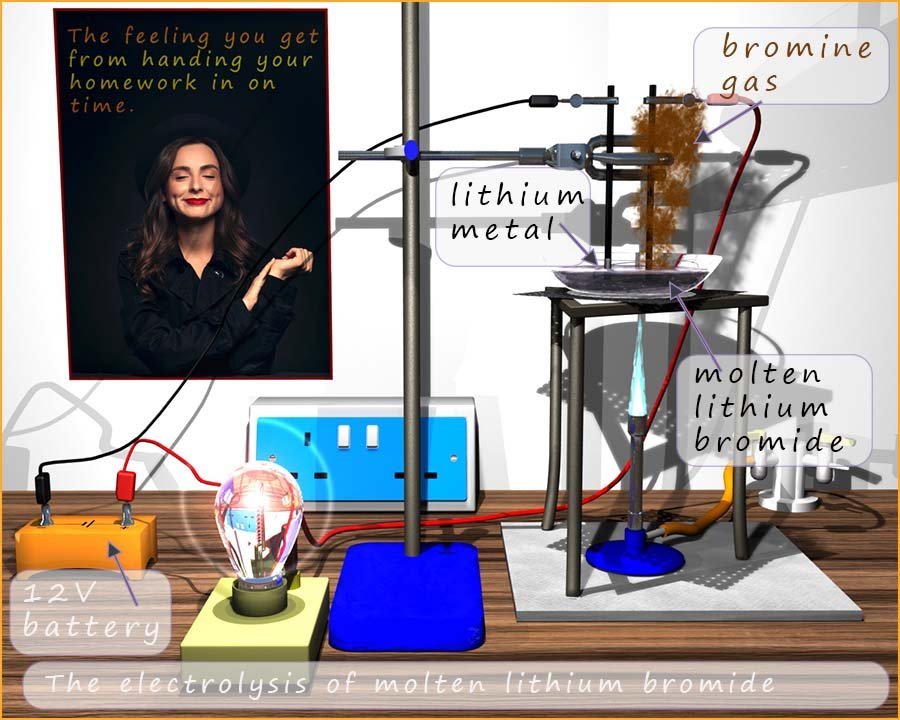
The image below outlines an electrolysis experiment that can be carried out in the lab to extract lithium metal from lithium bromide. Lithium bromide melts around 5500C, here it has been placed in an glass crucible and heated strongly with a Bunsen burner. Once it has melted two graphite electrodes are placed in the molten lithium bromide and an electrical current is passed through the molten lithium bromide. The electrical current will split the lithium bromide up into the elements that make it up, that is lithium metal and the non-metal bromine. You can see in the image that brown fumes of bromine gas are produced at the positively charged graphite electrode (the anode.)
A metal lower than hydrogen in the reactivity series can be
extracted from its ore; many of which are often simply metal oxides by simply heating with hydrogen gas, the hydrogen will essentially displace the less reactive metal from its ore/oxide. Copper for example can be extracted from copper oxide as shown below.
Here a stream of
hydrogen gas is passed over hot powdered copper oxide sitting
in a glass tube. The hydrogen being higher in the reactivity series than copper will remove or displace the oxygen from the copper
oxide. The hydrogen will reduce the copper oxide to copper metal. This is shown by the equations below:

The word and symbolic equation for this reaction are shown below:
If the metal is above hydrogen in the reactivity series
then it cannot be extracted from its ore by heating with
hydrogen. Instead the metal ore is heated to a high temperature with carbon or charcoal,
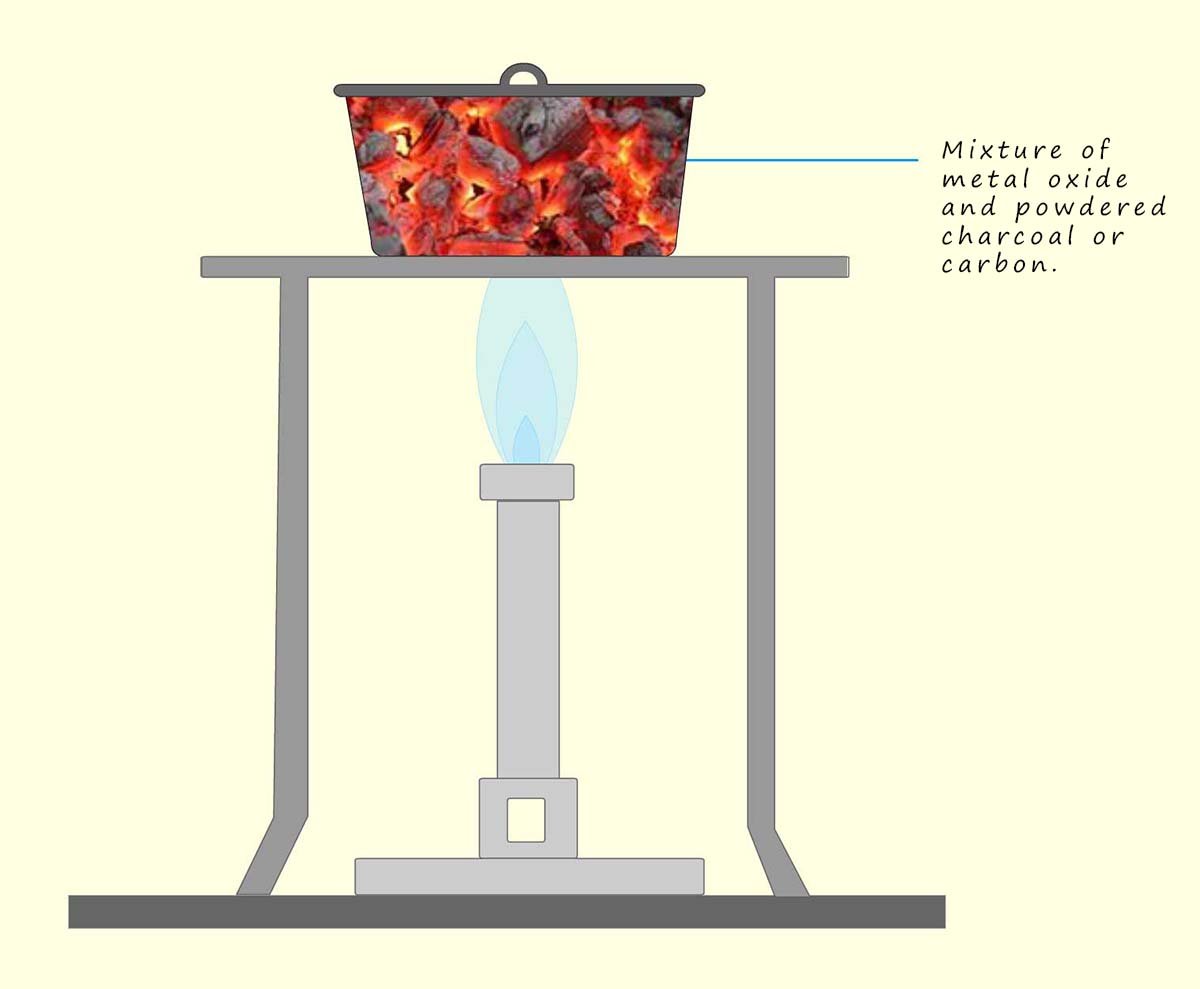 Carbon is a non-metal but it has been long used to extract
metals such as lead and iron from their ores. The process of extracting a metal from its ore by heating it to a high temperature in the presence of a reducing agent like carbon or charcoal is called smelting. The basic method outlined below show how a metal such as iron, tin, copper or lead can be extracted from its ore:
Carbon is a non-metal but it has been long used to extract
metals such as lead and iron from their ores. The process of extracting a metal from its ore by heating it to a high temperature in the presence of a reducing agent like carbon or charcoal is called smelting. The basic method outlined below show how a metal such as iron, tin, copper or lead can be extracted from its ore:
For example lead oxide, copper oxide and iron oxide can all be reduced by heating with carbon as shown in the diagram above. Equations for these reactions are shown below:
 Extracting metals from mines is not what you would call an environmentally friendly option! The image opposite shows a typical open cast copper mine. It uses large amounts of
energy and will destroy most if not all natural habitats. However the more we encourage recycling
metals then the more we can reduce the need for these mines.
Extracting metals from mines is not what you would call an environmentally friendly option! The image opposite shows a typical open cast copper mine. It uses large amounts of
energy and will destroy most if not all natural habitats. However the more we encourage recycling
metals then the more we can reduce the need for these mines.
There are more environmentally friendly ways to extract copper from its ore rather than using open cast mining. Copper is a very valuable metal which is in high demand. Most of the world's high grade copper ore has been used and so scientists have had to develop methods to extract copper from low grade ores; that is ores which may only contain a small percentage of the desired metal.
Using traditional methods to extract copper from low grade ores would potentially not be economically viable due to the large amount of copper ore/rock that would have to be dug up and processed to obtain a fairly small amount of copper. This would also produce a very large amount of waste that would have to be disposed of and ultimately put somewhere and this of course would lead to loss of land and habitat as well as incurring financial costs. However there are other ways to extract metals from their ores that do not require large amounts of energy or cause large amounts of environmental damage.
Use the slider below to give you a little more information about the method of extraction used for different metals and the energy requirements needed. The panel on the right hand side will give you some basic information about the methods used to extract metals. For the metals aluminium and iron click the links to visit the pages which will give you more detailed information. Why not do some further work yourself on electrolysis and find out how it is used to extract reactive metals such as sodium and potassium.
Answer the 5 questions below on metal extraction and then click the check answer button. If you get stuck use the reactivity slider above to get some help.
Pick the typical extraction method used for each metal. Use what you learned from the **carbon** and **hydrogen** lines in the slider activity above.

Plants have been used for many years to clean up land contaminated with heavy
metals such as mercury and lead.
Traditionally the contaminated soil would have be simply been scooped up by bulldozers and shipped elsewhere for disposal.
This is expensive and polluting. Plants can do a similar job but much more cheaply and in a less polluting and more
environmentally friendly and sustainable way.
Certain plants when grown on contaminated land will absorb the polluting heavy
metals into their roots and leaves, this will concentrate the metal in the plant cells and tissues. When
the plants are mature they can then be harvested, dried and then burned. The ash produced will contain the heavy
metal
compounds which can then be processed and the metals extracted. Using plants to extract metals
like this is called phytomining or phytoextraction.
Phytomining is often used to extract copper from low grade copper ores. Here the plants will be grown on the land containing the copper ore and when the
plants are fully grown they will be harvested, dried and burned as described above. The ash can then dissolved in water which is then acidified by adding dilute
sulfuric
acid to form an acidified copper sulfate solution. The copper can then be extracted from this solution by electrolysis or by carrying out a
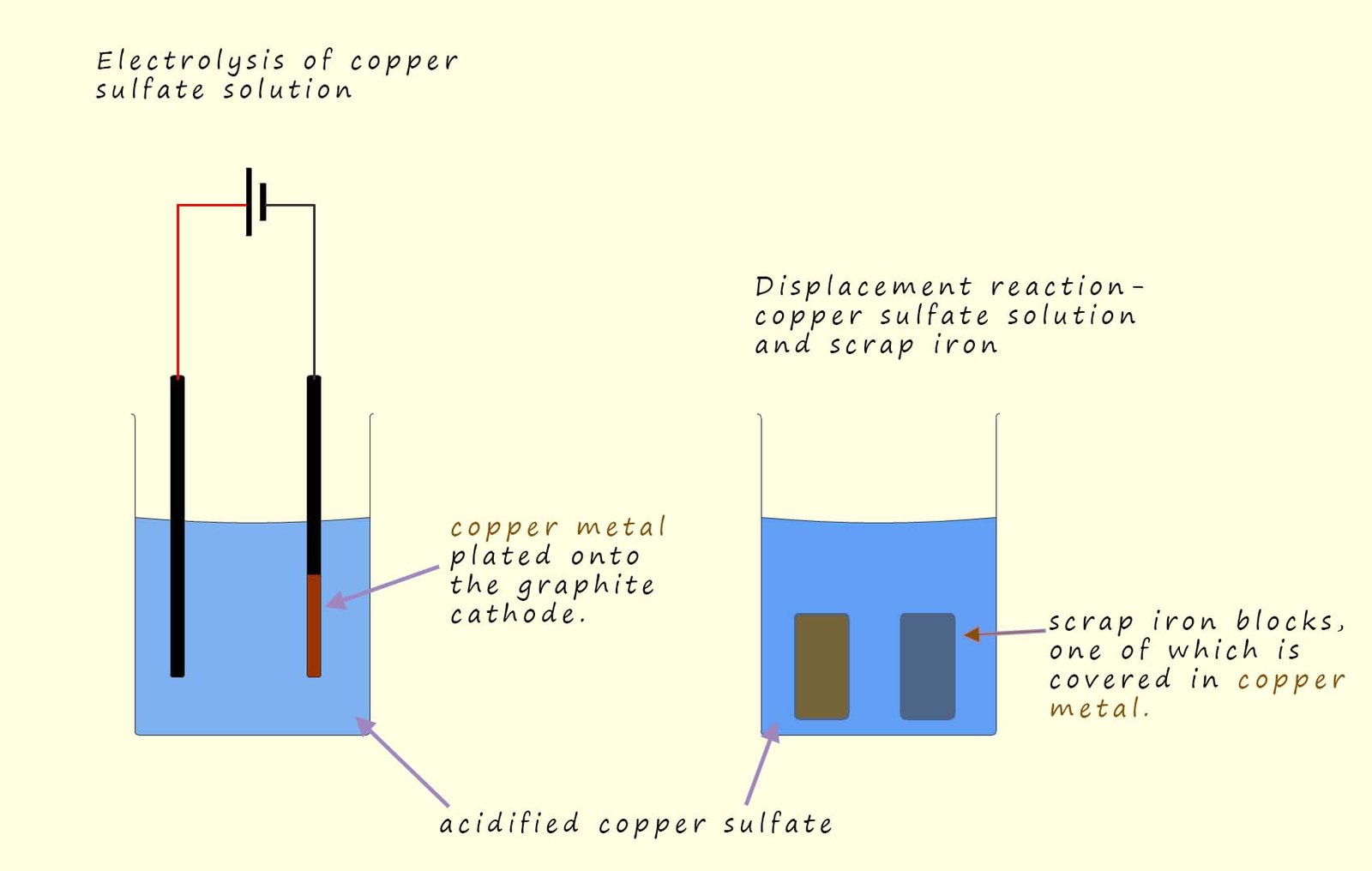
displacement reaction using scrap iron, as shown in the image below.
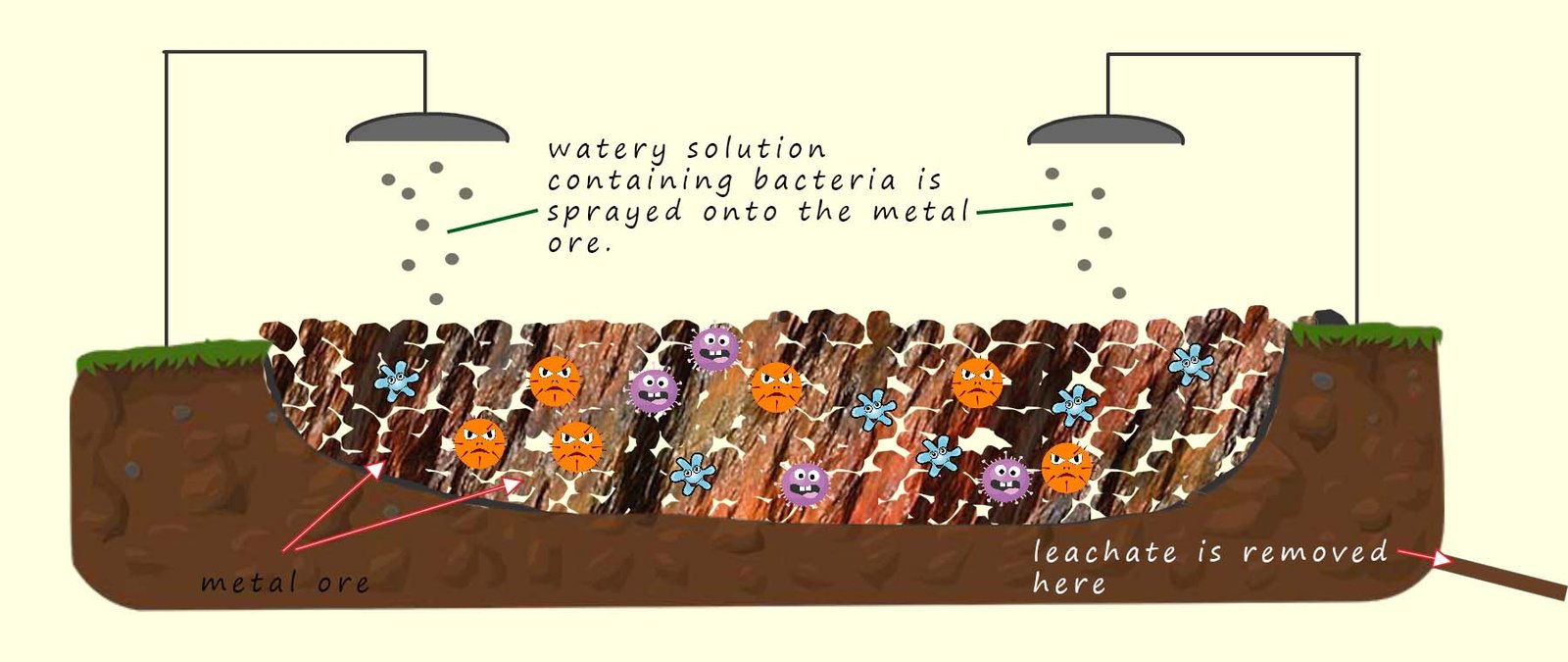
Bioleaching is another method that can be used to extract metals economically from low grade metal ores. Certain types of bacteria for example thiobacillus ferrooxidans are used in bioleaching. These bacteria feed on sulfide minerals present in low-grade copper ores, the bacteria produce a watery solution called a leachate which contains copper ions (Cu2+) through their metabolic processes.
Bioleaching is a very simple process; you can even buy kits to do it yourself on the internet! Basically a large hole is dug
in the ground and it is lined with a plastic liner. The metal ore is placed in the liner. The copper ore is sprayed with a watery solution containing the bacteria necessary to extract the metal from the ore. Many metals can be
extracted using this method including copper, nickel, zinc and uranium as well as many others.
This process is very
inexpensive and compared to traditional smelting methods where the copper ore is heated and reduced using charcoal or carbon and is much more environmentally friendly. The downside is that
it is very slow; it can take many months or even years to collect large amounts of metal. The diagram below shows just how simple and easy the process is to set-up with no specialist
equipment needed. Although if you decide to set-up your own bioleaching pit in the garden it is probably best to consult with your parents first!
The watery solution or leachate that collects at the bottom of the pit is simply collected and the
copper extracted by electrolysis or
displacement reactions; similar to the methods used to extract metals using phytomining.
Review your knowledge of bioleaching and phytomining by answering the questions below, use the hints below each question if you get stuck. Press the check answer button when your done.
Answer the questions on phytomining (phytoextraction) and bioleaching from low-grade ores.
| metal | method used to extract the metal from its ore |
|---|---|
| potassium | electrolysis |
| sodium | electrolysis |
| lithium | electrolysis of the molten compound |
| magnesium | electrolysis |
| aluminium | electrolysis |
| carbon | |
| zinc | heat the metal ore with carbon (smelting) |
| iron | heat the metal ore with carbon (smelting) |
| tin | heat the metal ore with carbon (smelting) |
| lead | heat the metal ore with carbon (smelting) |
| hydrogen | |
| copper | heat the metal ore with hydrogen or carbon |
| silver | often found as native metal; mild reduction/displacement |
| gold | found as a native element |
| platinum | found as a native element |
Test your understanding of the methods used to extract metals by completing the true or false activity below; simply drag the statements or click the statements and then a true or false bin. Press the check answer button when your done.
Drag statements into the bins or click a statement, then click a bin.