

This page will look at some of the use of nanoparticles, if you are not sure what nanoparticles are or what their common properties are then visit the page on nanotechnology for a quick recap.
Nanoparticles have many important applications in many different fields; here we will focus on the following uses of nanoparticles and we will look at both the advantages and disadvantages of this new technology in the following fields:
You are probably already familiar with the fact that nanoparticles are very small particles, typically they have sizes in the range of 1-100 nanometres and that many of their uses take advantage of two unique properties:
Catalysts are substances that speed up chemical reactions and the really cool thing is they do not get used up. Transition metals such as Manganese (Mn), copper (Cu), platinum (Pt), rhodium (Rh) and palladium (Pd) are commonly used as catalysts. The use of nanoparticles as catalysts means that the catalyst has a very large surface area making the catalyst more efficient, it also means that less of the expensive catalyst will be needed. Below are a few examples of where transition metal nanoparticles are used as catalysts:
 Let's be honest no-one enjoys cleaning an oven! Well with the recent advances made in the use of transition metal nanoparticles as catalysts the days of cleaning ovens are over! These self-cleaning ovens have a special liner coating the oven walls; this liner contains transition metal nanoparticles such as manganese oxide (MnO2) or copper oxide (CuO) which coat the internal walls of the oven.
These nanoparticles work through a process called catalytic oxidation; here is a breakdown of how they work:
Let's be honest no-one enjoys cleaning an oven! Well with the recent advances made in the use of transition metal nanoparticles as catalysts the days of cleaning ovens are over! These self-cleaning ovens have a special liner coating the oven walls; this liner contains transition metal nanoparticles such as manganese oxide (MnO2) or copper oxide (CuO) which coat the internal walls of the oven.
These nanoparticles work through a process called catalytic oxidation; here is a breakdown of how they work:
Titanium dioxide (TiO2) nanoparticles can act as photocatalysts, this simply means they use light, specifically ultraviolet (UV) light to activate their catalytic properties. These titanium dioxide nanoparticles are often used in water treatment works to drive chemical reactions that break down pollutants that are present in the water.
As an example consider the use of photocatalytic titanium dioxide nanoparticles that are used to keep the water in a garden pond clean.
 Here the titanium dioxide nanoparticles would be coated onto an inert ceramic material. If the inert ceramic material was then placed under a UV lamp and the water from the garden pond slowly pumped through it then the titanium dioxide nanoparticles would become activated.
Here the titanium dioxide nanoparticles would be coated onto an inert ceramic material. If the inert ceramic material was then placed under a UV lamp and the water from the garden pond slowly pumped through it then the titanium dioxide nanoparticles would become activated.
The titanium dioxide (TiO2) nanoparticles, when exposed to UV light ; either from sunlight or a dedicated UV lamp submerged or positioned near the pond can generate reactive oxygen species. These reactive oxygen species are usually reactive molecules called free radicals and they can help to:

Car exhausts contain harmful gases like carbon monoxide (CO), nitrogen oxides (NOx) and unburnt fuel or hydrocarbons. Catalytic converters help to turn these into less harmful substances such as carbon dioxide (CO2), nitrogen gas (N2), and water (H2O). The active catalytic materials inside the converter are often precious metals such as platinum (Pt), palladium (Pd) and rhodium (Rh). These metals are used in the form of nanoparticles with sizes in the range of between 2 and 10 nanometres, these nanoparticles are coated onto a ceramic honeycomb structure which itself has a very large surface area.
The metal nanoparticles in the catalytic converter have an incredibly high surface area compared to the same mass of the bulk metal. This means more of the active sites on the catalyst surface are available for the reactions to happen on, making the catalyst more efficient. Since the metal nanoparticles are so efficient, less of the expensive precious metals are needed to achieve the same catalytic effect, making this technology more affordable.
Traditional sun creams use larger particles of substances such as titanium dioxide (TiO2) and zinc oxide (ZnO) to block harmful UV radiation from the sun to protect the skin and prevent sunburn. However these larger particles often leave a white, chalky and greasy residue on the skin, which many people don't like.
However there is a solution to this problem and you may have guessed that it involves the use of nanoparticles! Modern sun creams often use nanoparticles of titanium dioxide and zinc oxide simply and because these nanoparticles are so incredibly small, they are transparent on the skin, eliminating the white chalky residue from traditional sun creams. The nanoparticles present in these sun creams still absorb and scatter UV rays, providing protection from the Sun without being visible on the skin. There is also the advantage that the tiny size of the nanoparticles allows for a more even and thorough spread of the protective minerals over the skin.
Body odour is something we probably don't want to talk about but we have all had it at some point! Deodorants and antiperspirants aim to control body odour caused by bacteria present on the skin breaking down sweat. Antiperspirants and deodorants work by trying to reduce the amount of sweat produced, as I am sure you are aware they are not always that successful!
However a better solution to body odour would be to kill the bacteria that interact with sweat and cause the body odour in the first place. Well some modern day deodorants do exactly that, they contain silver nanoparticles which are well known for their antimicrobial properties, that is they are able to kill or inhibit the growth of microorganisms such as bacteria and fungi; think of the silver nanoparticles present in deodorants as "little germ-zapper" on the surface of your skin preventing bacteria from multiplying and causing smells. The large surface area of the silver nanoparticles allows them to come into greater contact with bacteria on the skin's surface, inhibiting their growth or killing them and thus reducing unwanted body odours.
Traditional cosmetic ingredients often consist of larger, irregular-shaped particles; unfortunately these larger particles tend to clump together or settle unevenly on the skin's surface leading to streaks and patches and an uneven coverage, it is harder to get a thin, uniform layer with these larger cosmetic particles. These larger particles can make the product feel thick or drag on the skin, for example
these larger particles present in many traditional foundations and powders can sometimes sit on top of the skin's texture, exaggerating pores, fine lines, and dry patches, rather than creating a smooth, overall even finish.
 The presence of these larger particles also reflects light in an inconsistent way making the skin tone appear patchy or uneven.
The presence of these larger particles also reflects light in an inconsistent way making the skin tone appear patchy or uneven.
However nanoparticles with their incredibly small sizes (1-100 nanometres), can significantly improve the evenness of coverage in cosmetics. The ultra-fine size of nanoparticles allows cosmetic products to spread much more easily and evenly over the skin's surface, filling in tiny imperfections and creating a smoother base.
Nanoparticles blend seamlessly into the skin without leaving harsh lines or streaks because they distribute themselves uniformly.
When nanoparticles of minerals such as titanium dioxide are used in foundations, their small size helps to scatter light more effectively and evenly without leaving a visible white cast, contributing to a more uniform appearance.
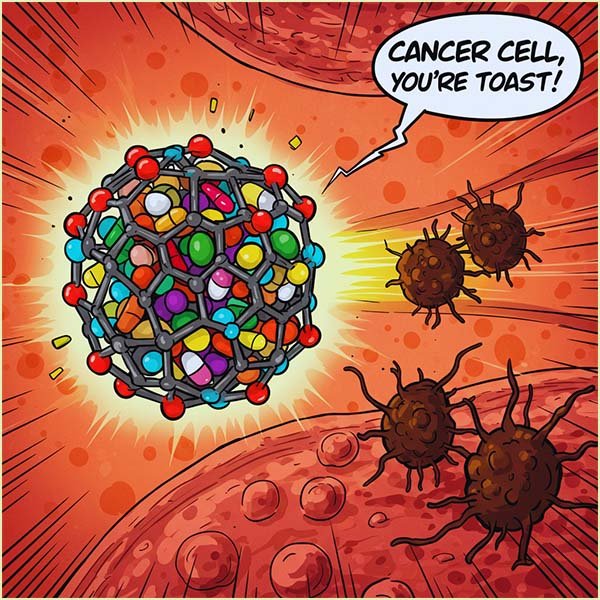 Buckyballs are shaped like tiny, hollow footballs made of carbon atoms. Many scientists are developing therapies that involve trapping certain drug molecules inside these football shaped buckyballs.
Buckyballs are shaped like tiny, hollow footballs made of carbon atoms. Many scientists are developing therapies that involve trapping certain drug molecules inside these football shaped buckyballs.
The surface of the buckyballs can be modified by attaching other molecules onto it and the clever part is that these molecules can be designed to specifically recognise and bind to proteins or other markers that are more common on the surface of tumour and cancer cells, it's like a GPS or satnav that guides the drug laden buckyballs straight to the cancer cell.
This "targeting" helps the buckyballs carrying the drug to stick to the tumour cells, delivering the medicine right where it's needed
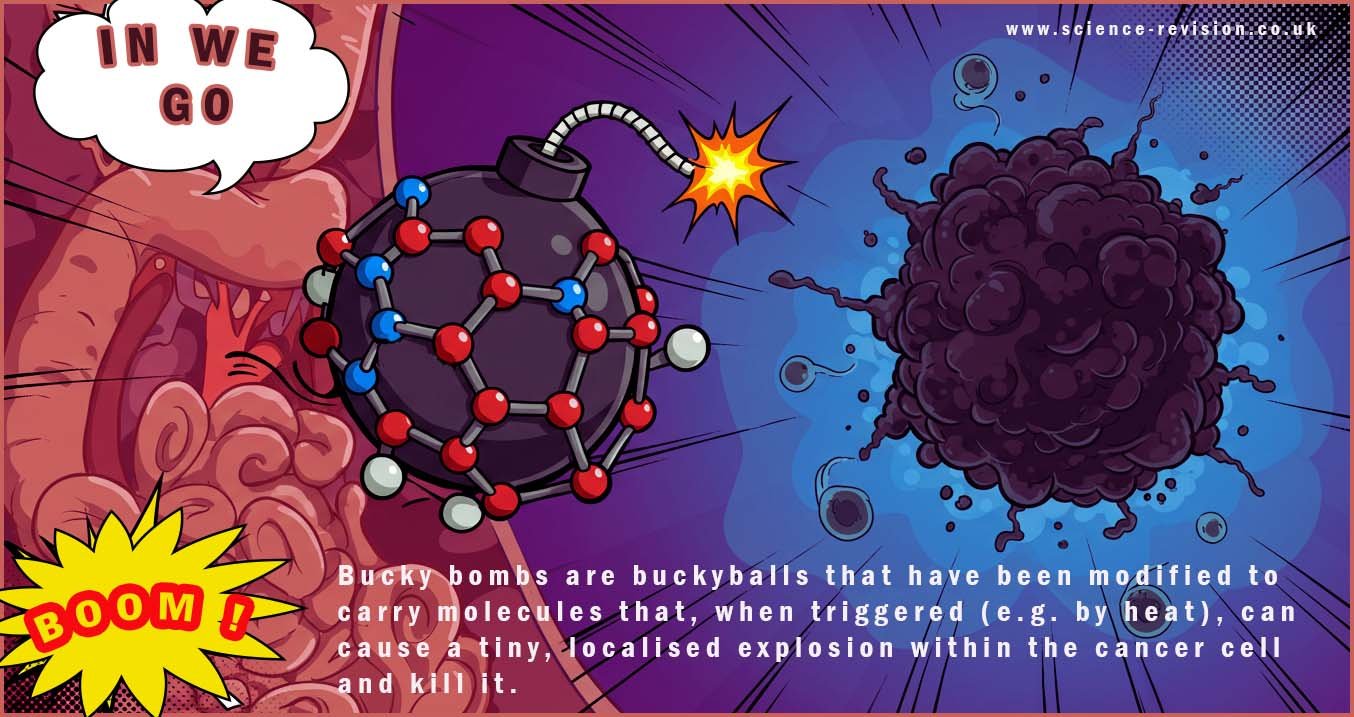
Some research involves placing what is in effect a "medical bomb or grenade" inside buckyballs. These buckyballs have been modified to carry molecules that when triggered for example by heat can cause a tiny localised explosion within the cancer cell, destroying it. Other areas of active research involves attaching buckyballs, each potentially carrying an active drug directly to antibodies. Antibodies are like the guided missiles of the body's immune system, in that they can recognise specific targets on cancer cells. This approach aims to deliver a high dose of the particular anti-cancer drug directly to the site of the tumor or cancer and destroy it leaving the surrounding normal healthy cells untouched.

Have you ever noticed that sometimes when you take off a sweater or your PJs that you can get a small electrical shock that can make your hair stand on end? Well some clothes; especially those which have nylon or polyester in them can build up and store a small static charge. Well if the material used in clothing contains zinc oxide or titanium dioxide nanoparticles this will prevent the build up of this static charge and stop these small electrical shocks!
Many brushes and in particular hair brushes are another common and also rather annoying area where static electricity can build up. Have you ever brushed your hair and noticed that your hair sticks to the brush and your hair is all frizzy after brushing? Well this is due to the build up of static electricity due to the friction caused by simply brushing your hair.
However as before nanoparticles can come to the rescue here! Carbon nanotubes for example can help to transfer static charge away from the hair as you brush and eliminate this problem. Some researchers have even created brushes with bristles made entirely of carbon nanotubes. Silver nanoparticles can also be incorporated into the brush material or bristles, these will increase electrical conductivity and reduce static with the added advantage that silver nanoparticles also offers antimicrobial benefits for the brush itself.
Silica nanoparticles when applied to clothing will help make it stain and waterproof. The silica nanoparticles will repel tea, coffee, wine and even oils, so you would not need to worry about getting soaked through the next time you got caught in a heavy shower of rain. The silica nanoparticles will also repel sweat so no need to worry about embarrassing sweat stain under your arm pits on a hot summers day! An application of silver nanoparticles would also kill bacteria which would also mean that your clothes won't smell either, this would also be ideal for sportswear. An application of titanium dioxide nanoparticles and an acidic catalyst can make your clothes wrinkle free so no need to worry about ironing your trousers or shirts anymore!

Carbon nanotubes can be woven into cotton and other materials to produce a fabric that will conduct electricity. These fabrics could then have sensors implanted in them to monitor such things as heart rate, blood pressure or sugar levels, it also allows the possibility of "wearable tech" with other devices and electronic gadgets being woven into our clothing.
Transistors are tiny switches that control the flow of electricity in computer chips. Nanoparticles and nanomaterials like graphene and carbon nanotubes can be used to make these transistors much smaller than traditional ones. Smaller transistors means that they can be packed more densely onto a computer chip, leading to faster processing speeds and ultimately more powerful and smaller computers and electronic devices.
Quantum dots are tiny semi-conductor nanoparticles that glow with specific colours when light shines on them or when electricity passes through them. These quantum dots can be used in LED displays to produce purer and more saturated colours, leading to brighter and more vibrant screens on all our electronic devices. They will also be more energy-efficient than traditional display technologies.
Nanomaterials can be used to improve the efficiency of solar cells in converting sunlight into electricity.
Nanoparticles can increase the amount of sunlight absorbed by the solar cell.
They can also help to improve the flow of electrons, increasing the electrical output of the solar cell.
Finally nanomaterials like graphene and carbon nanotubes are very strong and flexible and they can conduct electricity.
This allows for the creation of electronic components and circuits that can be bent, folded, and stretched without breaking, leading to the development of flexible screens and wearable electronics. Imagine rolling up the TV and putting it in the drawer when you have finished watching it or rolling up your phone and slipping it into your wallet or purse!
These are only a few of the possible uses of nanoparticles. This is a field of chemistry which will only grow in the future as more research into the uses of these new materials is carried out.

Many of the ideas and uses of nanoparticles discussed above suggest that this new technology is going to improve all our lives and help cure many serious diseases and illnesses, however with any new technology there is likely to be areas or concern and potential risk due to the unknown problems that this new technology may cause; for example: