

Higher and foundation tiers
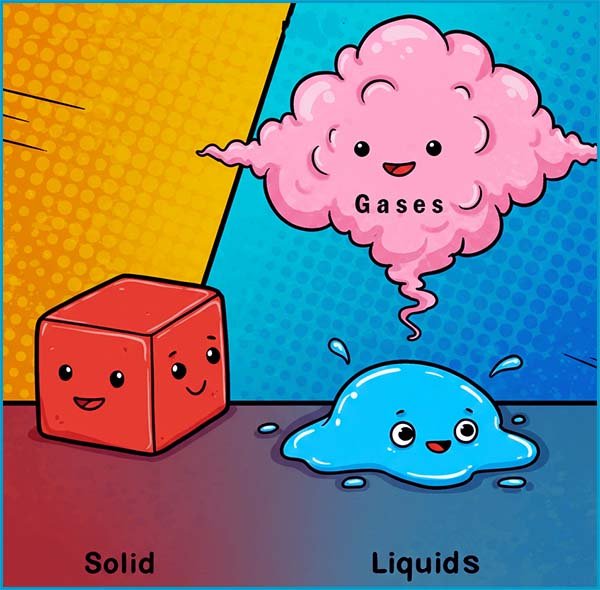
The particle model as its name suggests is a model or theory used by scientists to explain many of the physical properties of matter (solids, liquid and gases). The particle theory or model assumes that all matter is made up of particles and that these particles:
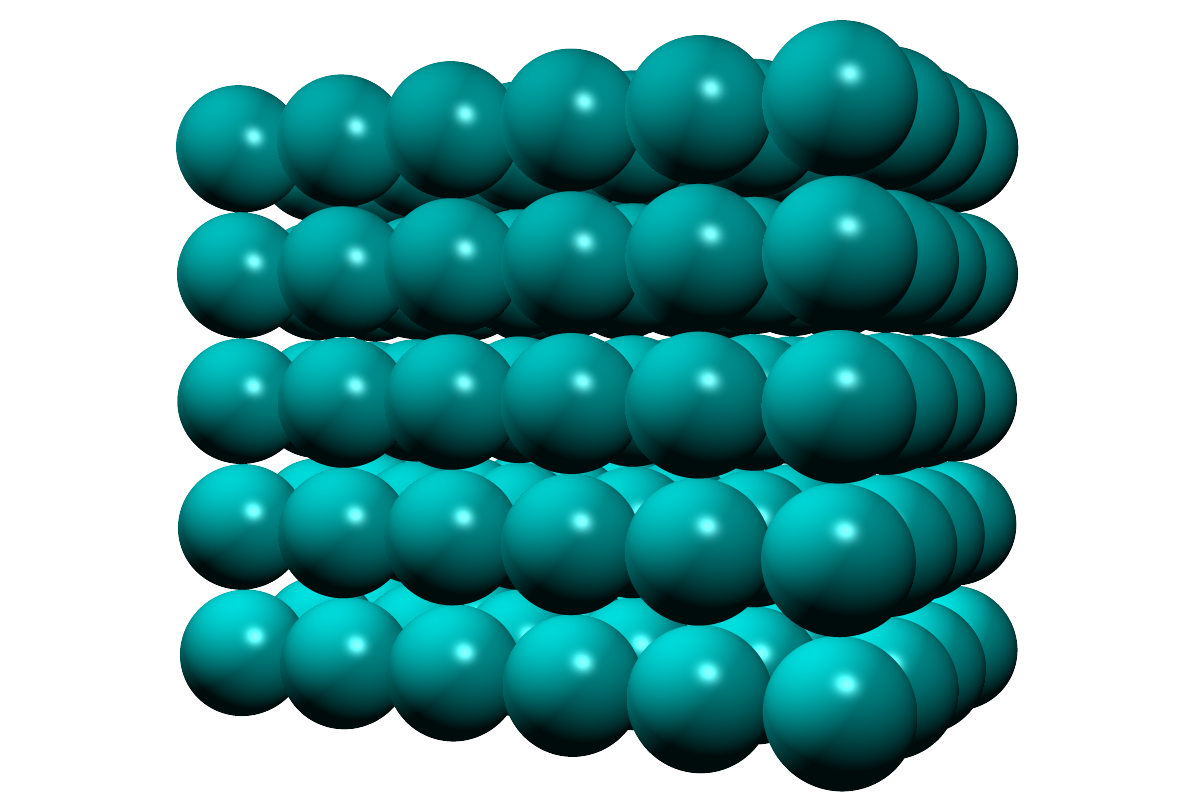 In a solid:
In a solid:
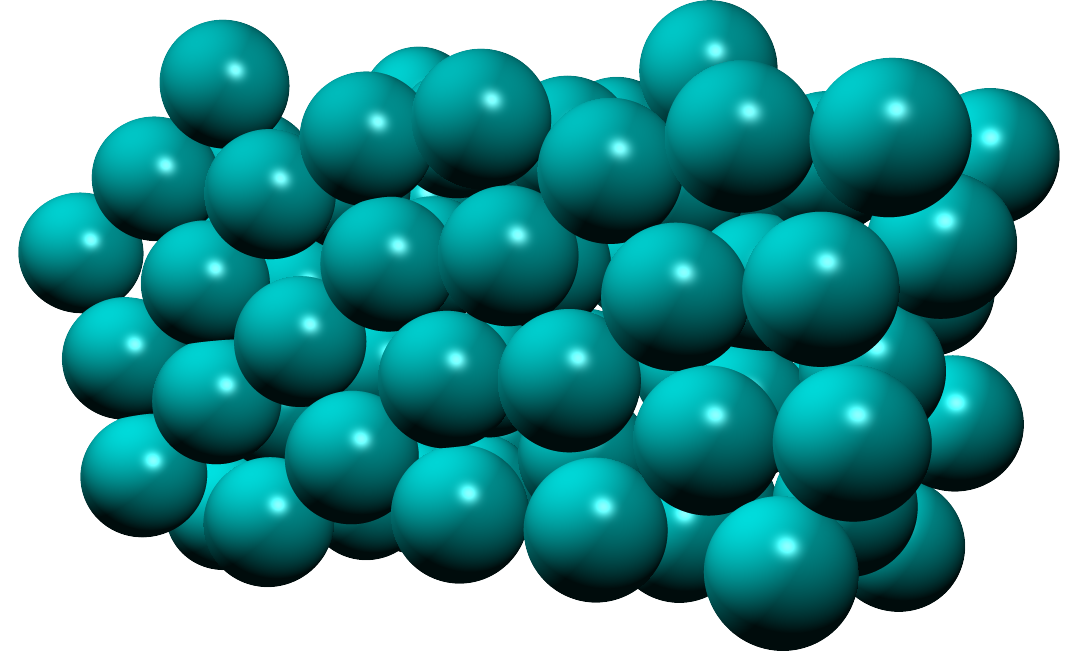 In a liquid:
In a liquid:
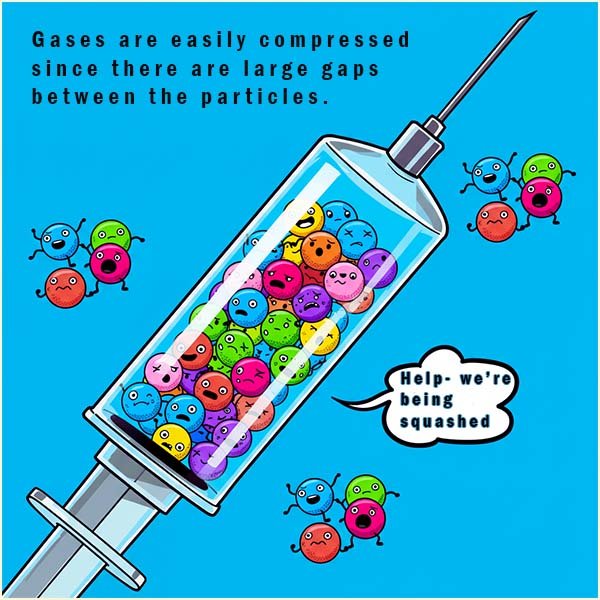
In a gas the particles:

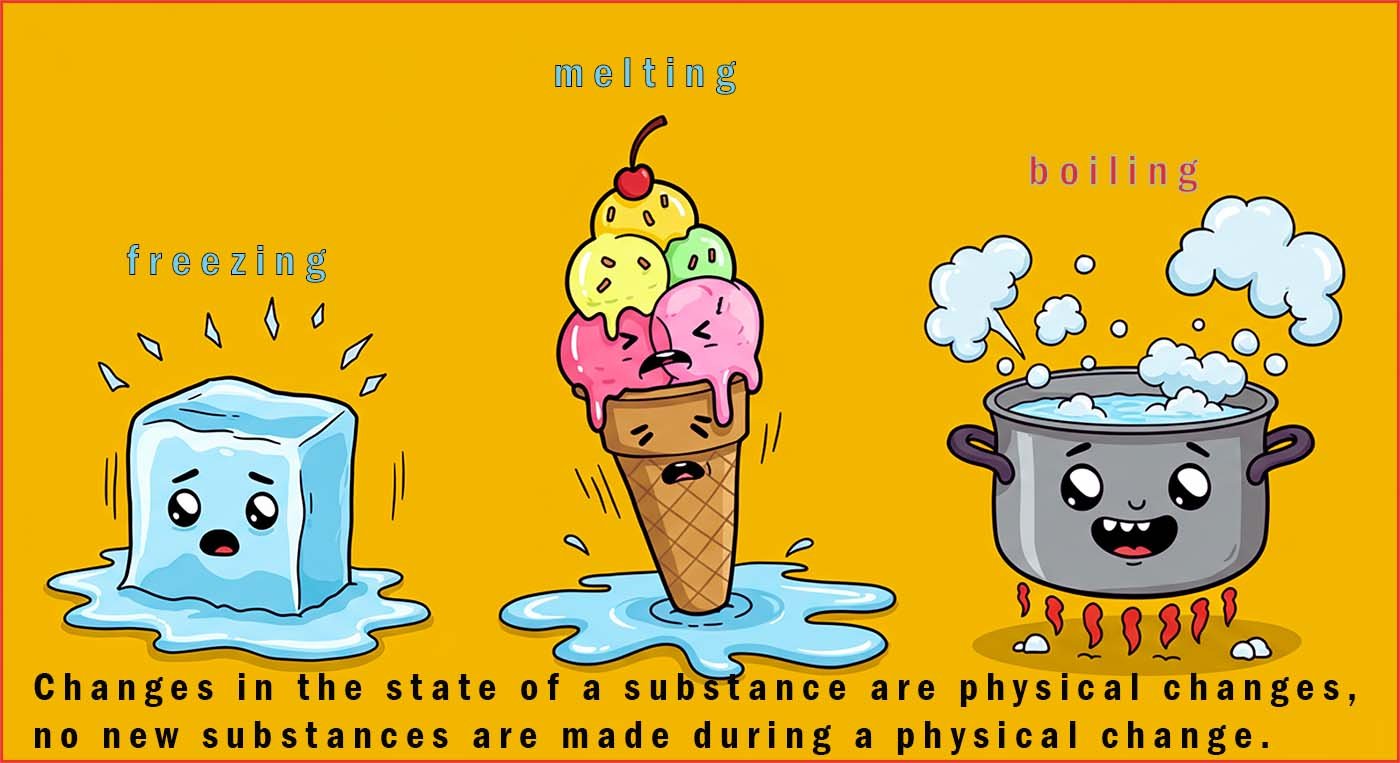
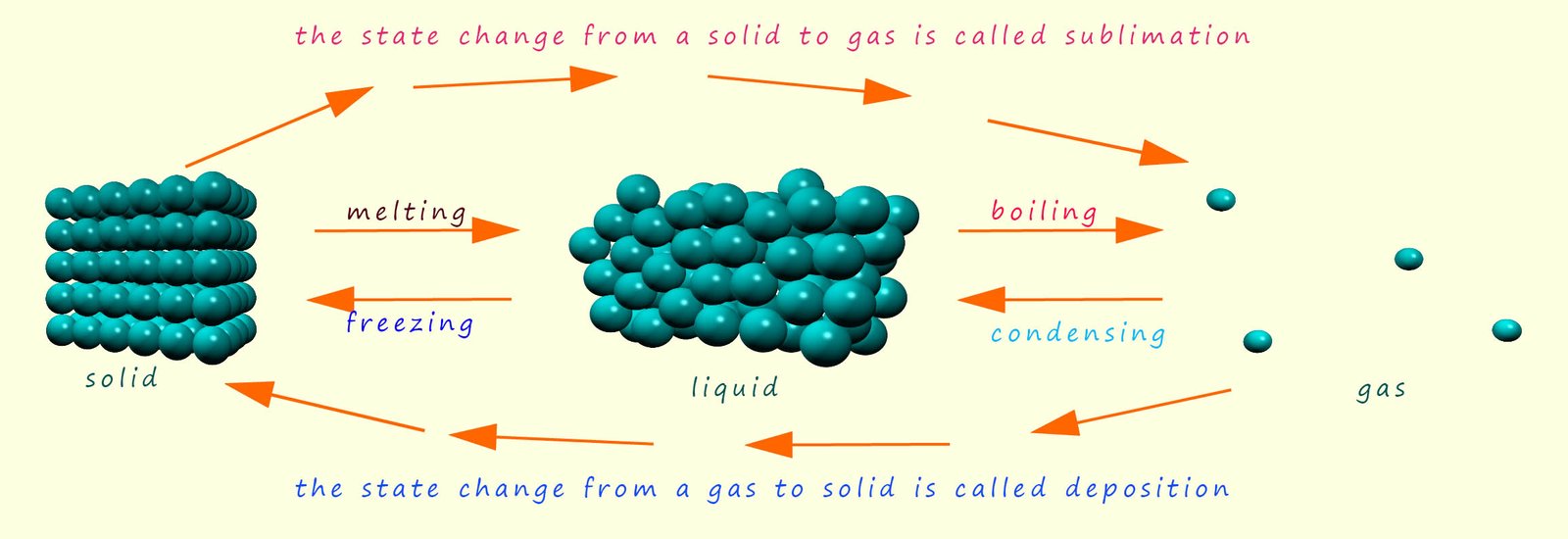
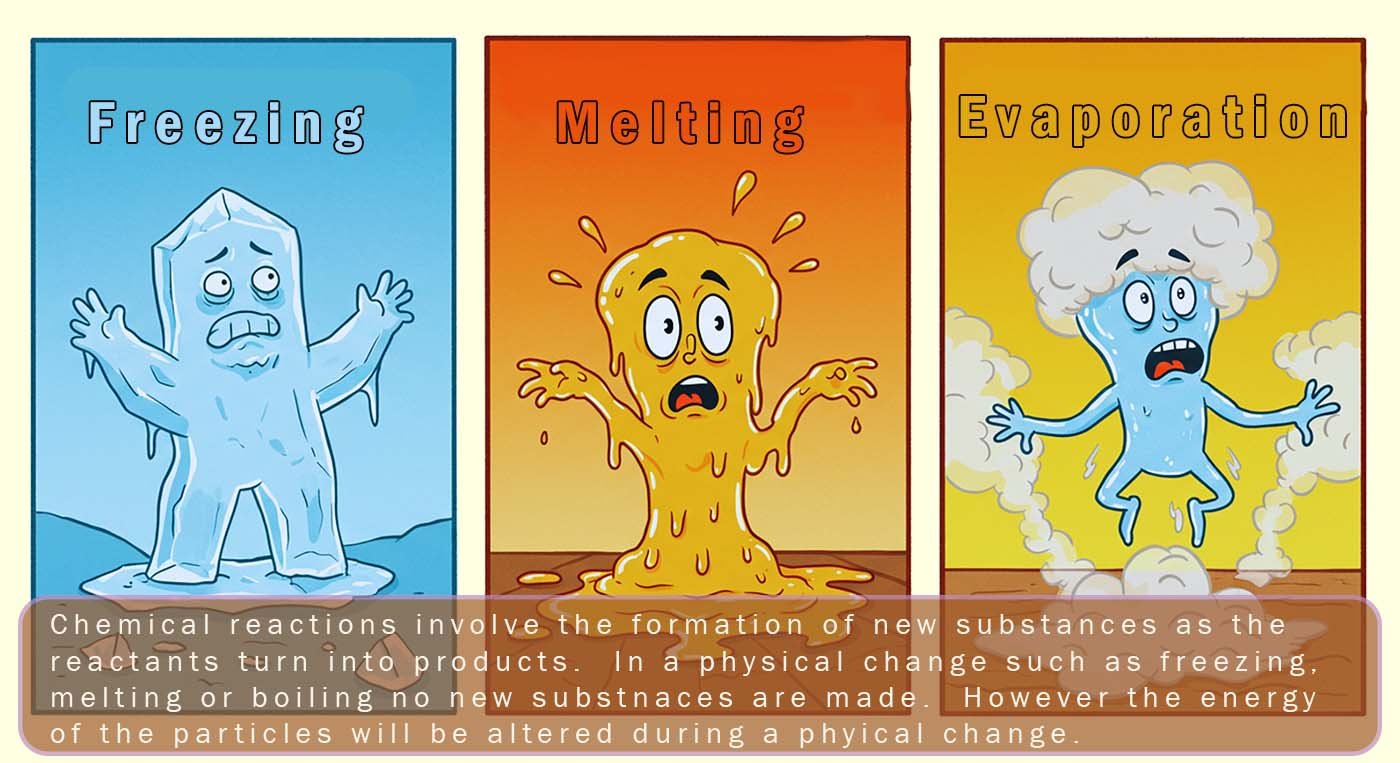
During a chemical change (a chemical reaction) new substances are made; the
reactants turn into products. During a
physical change no new substances are made. The
particles simply rearrange themselves by losing or gaining energy e.g.
melting, freezing and boiling are all physical changes.
 To predict the state of a substance at a certain temperature
is not always as straight forward as it seems; especially
when the melting and boiling points are very low e.g. nitrogen gas has a melting point of -2100C and a
boiling point
of -1960C . To help you predict the state of a substance at any given
temperature carry out the following quick steps:
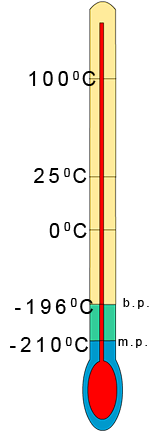
To predict the state of a substance at a certain temperature
is not always as straight forward as it seems; especially
when the melting and boiling points are very low e.g. nitrogen gas has a melting point of -2100C and a
boiling point
of -1960C . To help you predict the state of a substance at any given
temperature carry out the following quick steps:
Like most scientific model the particle model is not perfect; it has its good and bad points. However some of the assumptions that it makes are not always valid:
 In liquids the particles are close but can move and slide past each other randomly. Liquids have a fixed volume but take the shape of their container and are also incompressible.
In gases the particles move very fast and are far apart with weak attractions between the particles. Gases fill their container and have no fixed shape or volume, and they are easily compressed.
In liquids the particles are close but can move and slide past each other randomly. Liquids have a fixed volume but take the shape of their container and are also incompressible.
In gases the particles move very fast and are far apart with weak attractions between the particles. Gases fill their container and have no fixed shape or volume, and they are easily compressed.