
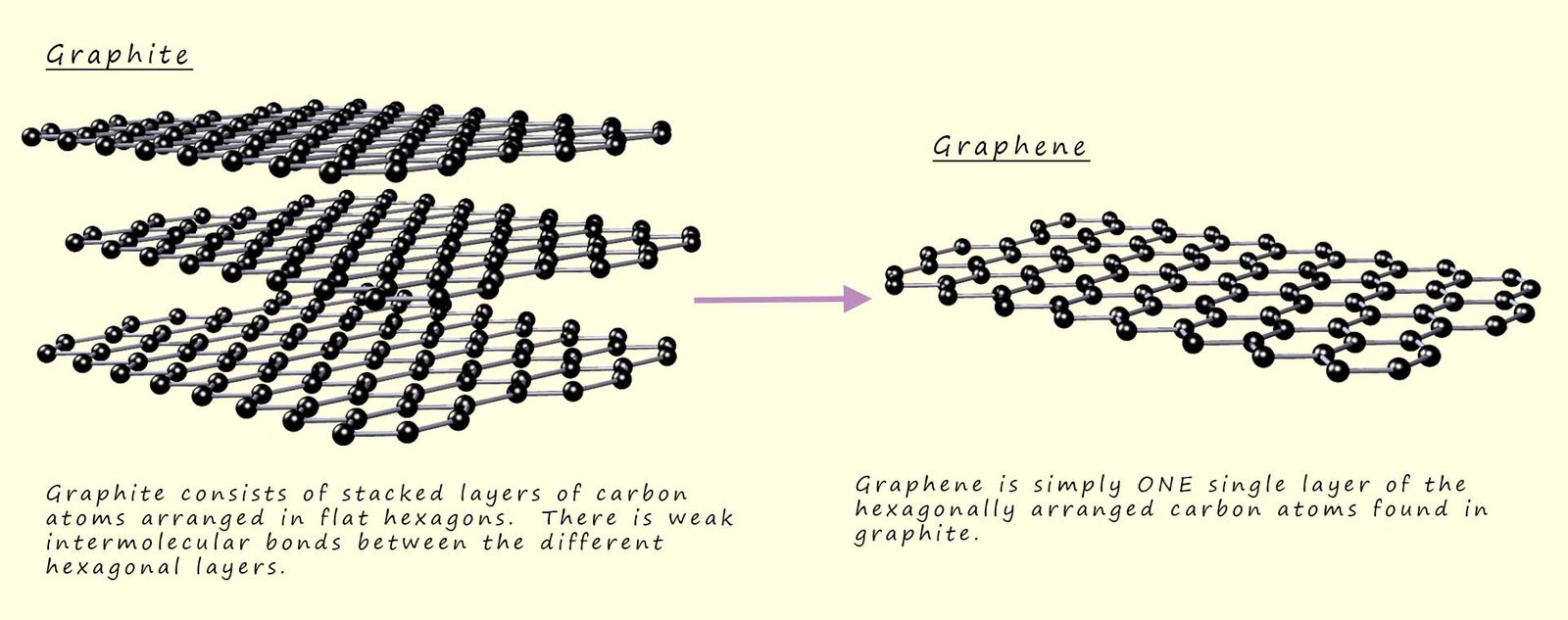
Graphene is another allotrope of the element carbon and a newly discovered material which was first isolated in 2004. Graphene was first isolated from graphite, you may recall that graphite consists of stacked layers of carbon atoms arranged in layers of hexagons as shown in the image below. Each of these individual layers of hexagonal carbon atoms is in fact graphene! There only weak intermolecular forces between each of these layers of graphene or carbon atoms in graphite and so each of these layers can be easily separated from each other. The first samples of graphene were obtained by removing these single layers of these carbon hexagons from graphite using some very hi-tech stuff- sellotape!!
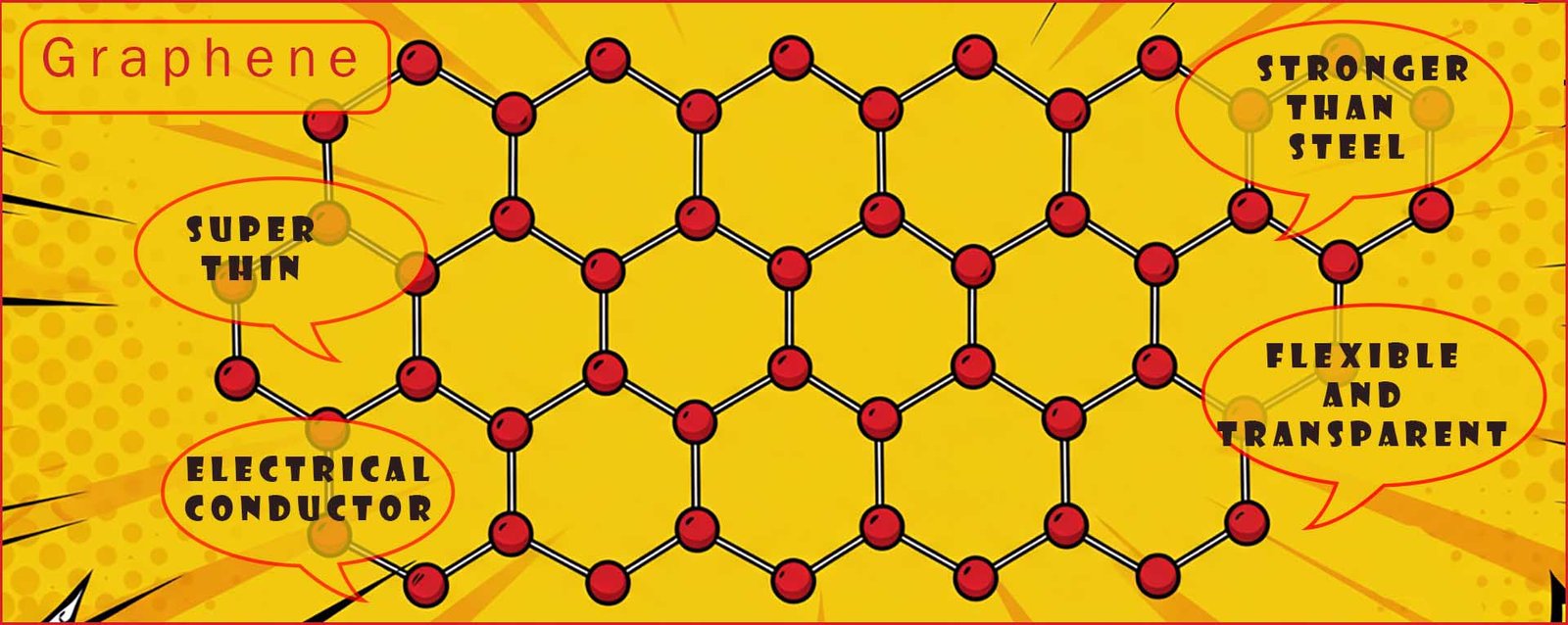
Like graphite each carbon atoms in graphene forms three strong covalent bonds leaving one spare free electron. These free moving delocalised electrons are responsible for the excellent electrical conductivity of graphene. The three strong covalent bonds made by each carbon atom make graphene an incredibly strong material despite the fact that each graphene layer is only one atom thick; in fact each graphene layer is some 200 times stronger than steel by weight. The image below shows the individual layers or hexagon lattice of carbon atoms that makes up each graphene layer.

Graphene is one of the strongest substances known despite only being only one atom thick; it is an excellent thermal (heat)
 and electrical conductor, it is transparent and highly flexible. These properties seem like a scientists dream material
and open up graphene to a mass of uses. Possible uses include:
and electrical conductor, it is transparent and highly flexible. These properties seem like a scientists dream material
and open up graphene to a mass of uses. Possible uses include: