

Before we dive into the equilibrium topic in A-level chemistry, let’s recap the core principles of equilibrium you learned at GCSE.....
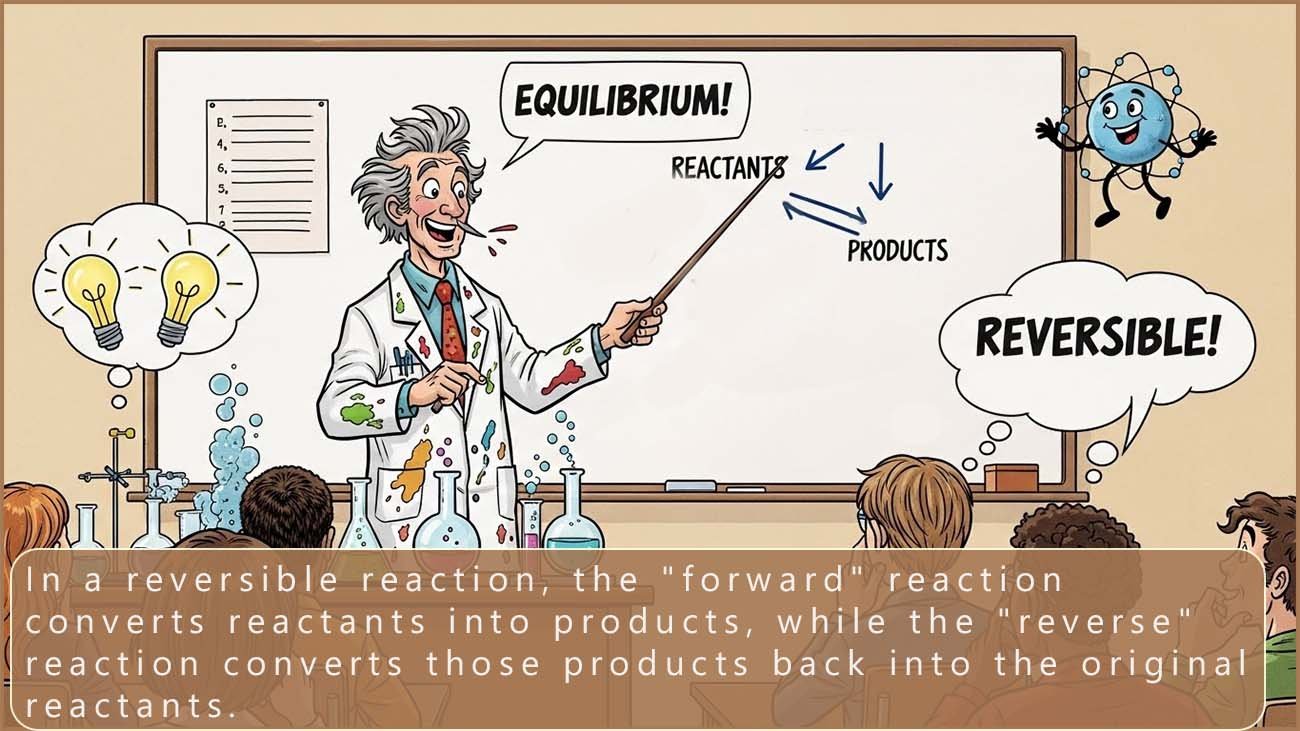
In some chemical reactions the reactants form products and at the same time the products can react to reform the reactants. This means two reactions are happening at the same time, but these two reactions are taking place in opposite directions. These reactions are called reversible reactions.
The equation below can be used to represent a reversible reaction:
The arrow pointing right shows the forward reaction (reactants forming products). The arrow pointing left shows the reverse reaction (products reforming reactants).
Quick check: If two opposite facing arrows are present in a chemical equation, does it mean:
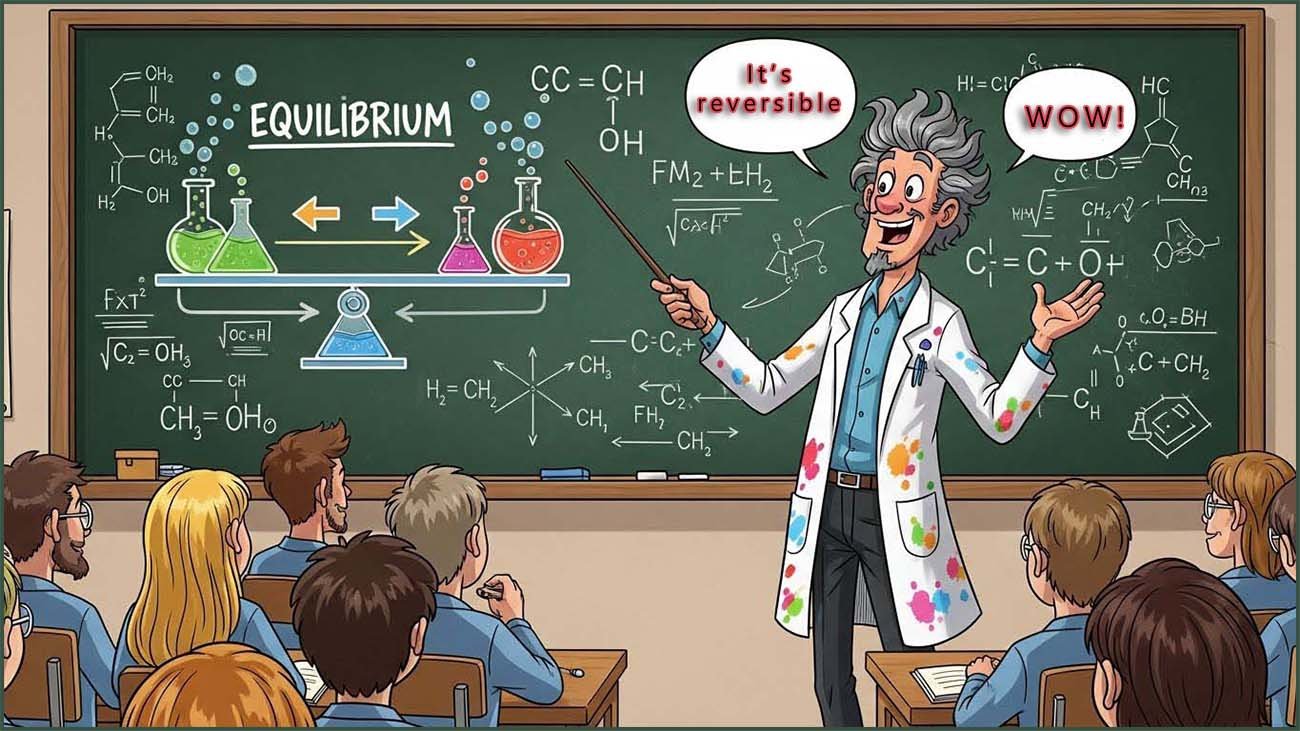
In a reversible chemical reaction where the forward and reverse reactions are both happening at the same time the rates of both of these reactions do not have to be the same. It is a common misconception that the rates of these two reactions need to be the same, they rarely are.
Since both the forward and reverse reactions are happening at the same time, the amounts of reactants and products that are present at any one time will be constantly changing.
Think about this:
If the forward and reverse reactions are happening at the same time, but the forward reaction is faster, what will happen to the amounts over time?
As a reversible reaction continues it eventually reaches a point where the rate of the forward reaction equals the rate of the reverse reaction. At this point, the concentrations or amounts of reactants and products present stay constant; they do not change even though both the forward and reverse reactions are both still happening. This state is called dynamic equilibrium.
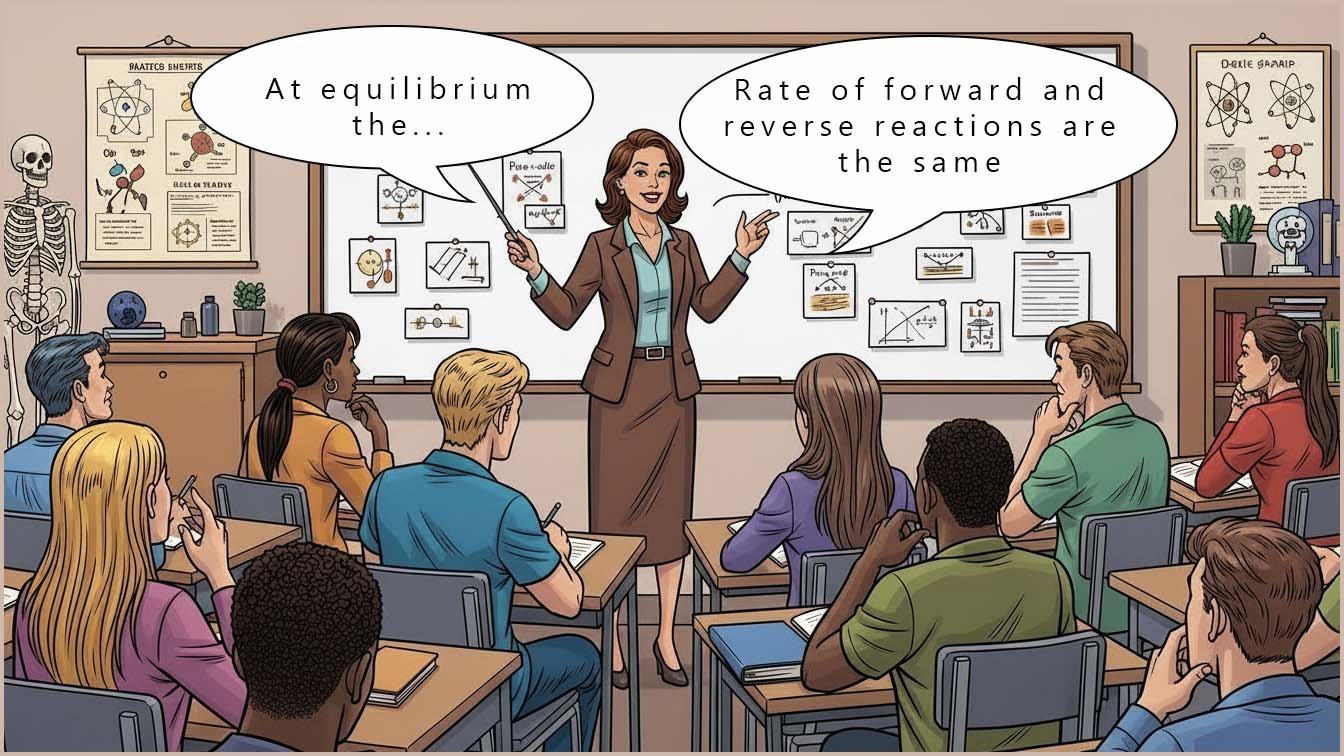
The word dynamic means the reactions are still occurring. The word equilibrium means the forward and reverse reactions are happening at the same rate.
Quick check:
At dynamic equilibrium, which statement is correct?
When students first meet the idea of equilibrium in chemical reactions, several incorrect ideas or misconceptions are very common. Clearing these up early makes everything that follows much easier to understand e.g.
A good way to think about equilibrium is as a situation where change is still happening, but no overall difference can be seen.
Not all reversible reactions ever reach equilibrium. For equilibrium to be established, the reaction must take place in a closed system. A closed system is one in which no matter can enter or leave. This is essential because equilibrium depends on the amounts of reactants and products remaining constant.
If any substance is able to escape from the reaction mixture; for example a gas leaving an open flask or beaker then the amount of that substance will obviously decrease and as a result the rates of the forward and reverse reactions will never be able to balance and the reacting system (chemicals) will not be able to achieve equilibrium. The image below shows a simple example of an open and closed system; the reversible reaction taking place in the open conical flask will never achieve equilibrium simply because matter can be seen leaving the flask while the reaction taking place in the closed system (flask) will eventually reach equilibrium.

Key idea:
Dynamic equilibrium is only possible in a closed system.
If any reactant or product can escape or be added, equilibrium cannot be achieved.
This explains why many laboratory reactions appear to go to completion. If a gas is produced and allowed to escape, the reverse reaction becomes impossible, even if the reaction is reversible in principle.
Check: Would a reversible reaction ever reach equilibrium if one of the products is a gas and the container is left open?
Equilibrium can also be represented using a graph. This helps to show clearly how the amounts of reactants and products change over time and it also highlights what stays the same.
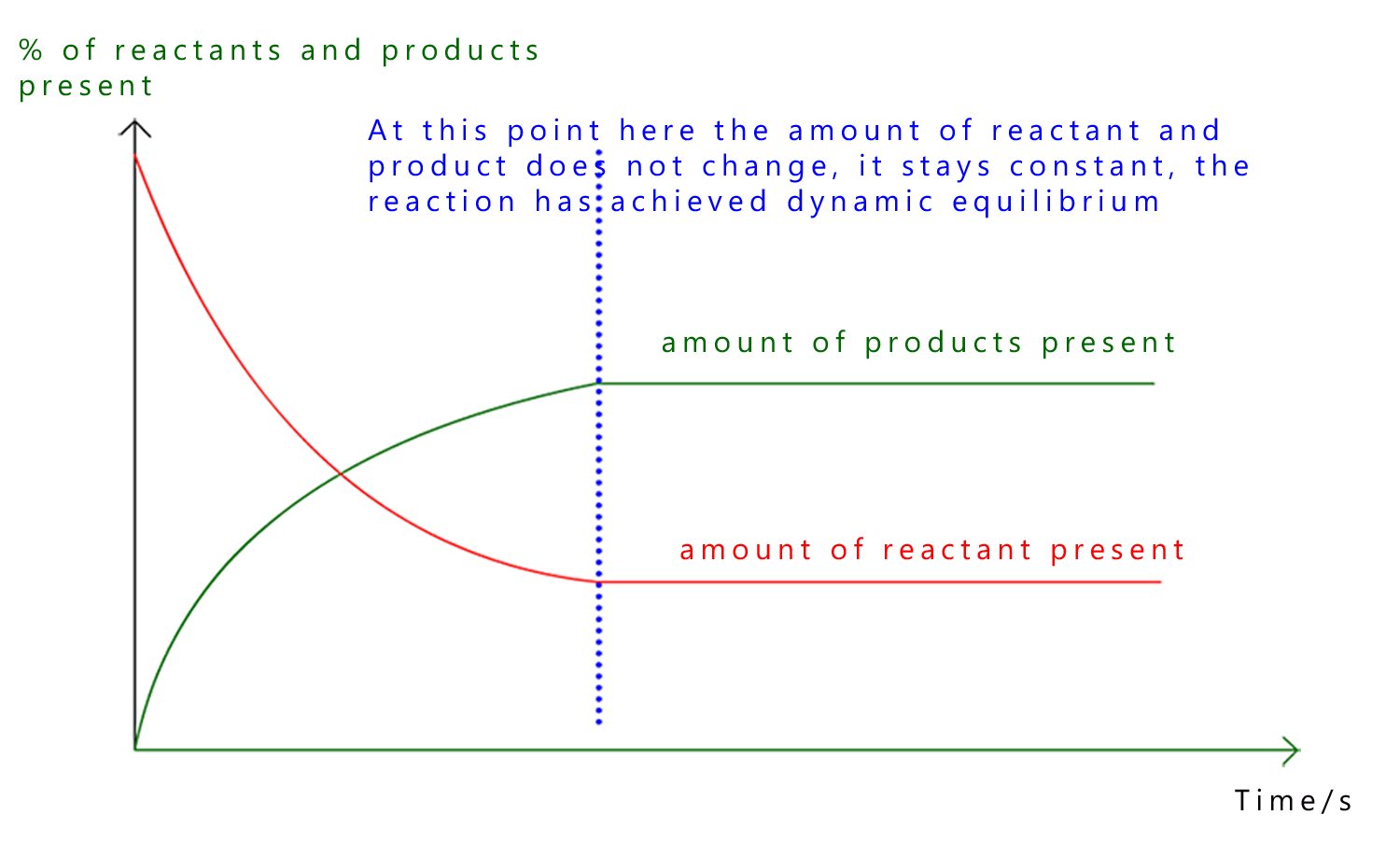
At the start of the reaction there is a large amount of reactants and little or no product. As the reaction proceeds the reactants are used up and products are formed, this is outlined in the graph above.
However eventually both lines level off, this is the point at which the reaction has reached dynamic equilibrium, here:
Even though the lines on the graph are flat the reaction has not stopped. Particles are still reacting in both directions but there is no overall change in the amounts of reactants and products present.
Many reversible reactions that you are likely to meet involve simple physical or chemical changes, for example two common lab examples are shown below. You may have carried out these two chemical reactions or at least seen them in the lab!
Copper sulfate is a colourless (white) ionic crystalline solid. However if you have a bottle of copper sulfate in the lab it will be blue and not white (colourless). The reason for this is that the colourless anhydrous copper sulfate absorbs water from the air and this turns it blue. The dry or anhydrous copper sulfate has the formula CuSO4; it is an ionic compound with a giant ionic lattice structure. The water it absorbs from the air fits into this lattice structure and is held weakly in place. The wet or hydrated copper sulfate has the formula CuSO4·5H2O (the ·5H2O just means that 5 moles of water are associated with its crystal structure). This water can be easily evaporated from the lattice by simply heating it. This is shown below; once it has evaporated it loses its blue colour and turns white or colourless to form anhydrous copper sulfate. Addition of water again forms the hydrated blue form of copper sulfate. This is outlined in the image below:
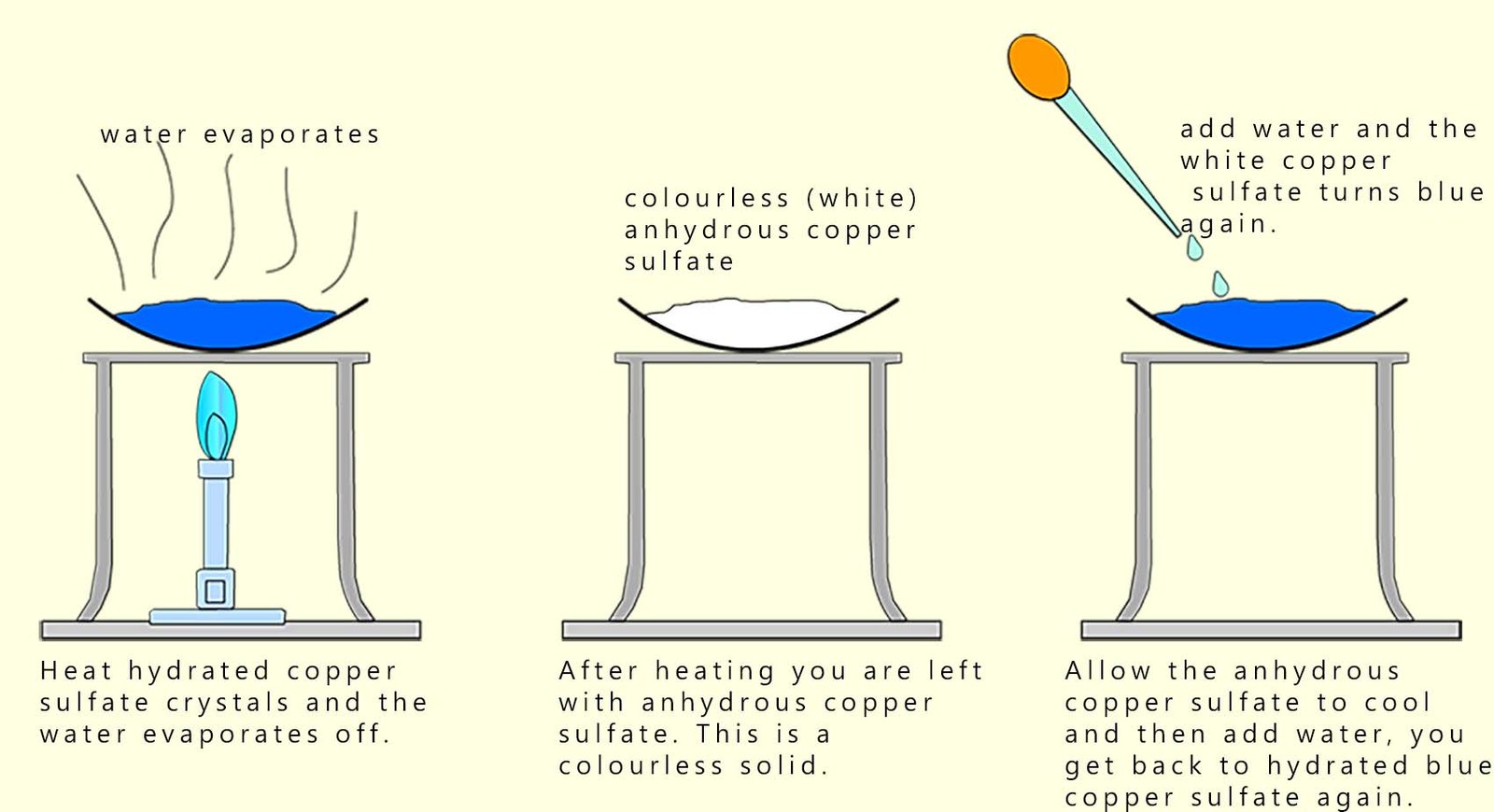
Heating hydrated copper sulfate removes the water, forming the anhydrous solid again. Adding water reverses the change.
This reaction is reversible but it does not reach equilibrium because the water vapour can escape to the surroundings, that is the reaction is not carried out in a closed system.
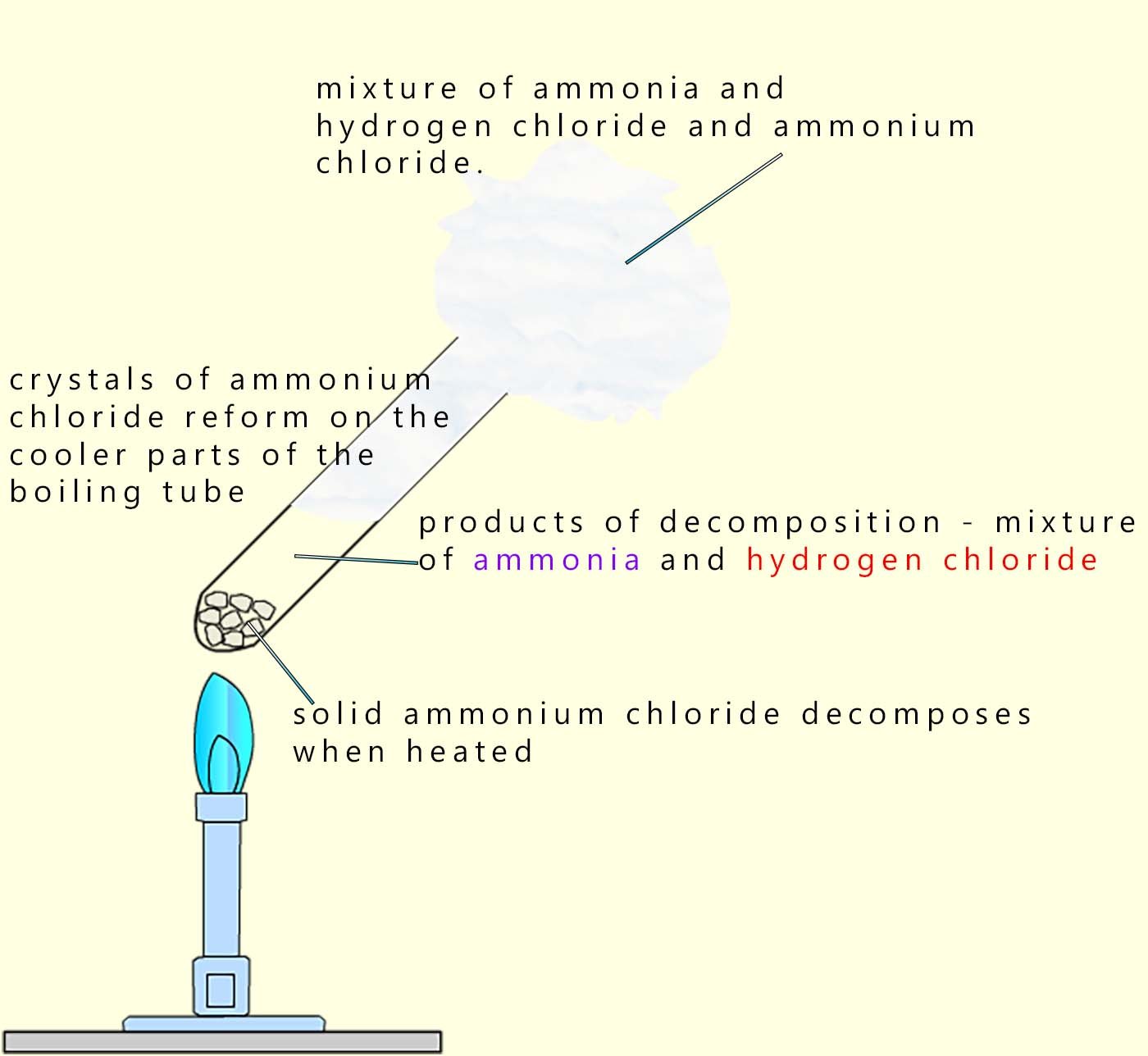
Ammonium chloride is a colourless solid which will decompose when heated to form a mixture of two gases; ammonia and hydrogen chloride gas. This reaction is reversible and when cooled the mixture of the basic ammonia and the acidic hydrogen chloride gases will react and reform solid crystals of ammonium chloride. This reaction can be shown as:
Although this reaction is reversible; equilibrium is not reached in an open system because the gases are free to escape.
In exam questions marks are often lost because key ideas are confused. The points below summarise exactly what examiners expect.
Tip: If a question mentions equilibrium, look for clues about whether the system is closed.