


Aromatic compounds containing benzene rings are susceptible to attack by electrophiles, that is electron deficient or electron poor species. Common electrophiles include positively charged ions such as: hydrogen ions (H+) and nitronium ions (NO2+) as well as molecules with polar covalent bonds which contain atoms which carry a partial positive charge such as molecules containing the carbonyl group (C=O).
Aromatic compounds containing benzene rings have areas of high electron density or to be more accurate molecular orbitals containing delocalised pi (π) electrons above and below the flat plane of carbon atoms; as shown in the image opposite. These clouds of electron density attract electrophiles, which will act as electron acceptors and readily form bonds with the delocalised pi(π) electrons in the benzene ring.
The molecular formula of benzene (C6H6) would suggest that it is an unsaturated molecule and therefore you might expect it to undergo electrophilic addition reactions which are typical of unsaturated molecules such as the alkenes. However you may recall that the presence of a delocalised system of pi (π) electrons within benzene rings adds extra stability and makes benzene and other aromatic molecules much more stable that might be expected. This delocalisation energy (also called resonance energy) is significant being approximately 152 kJ/mol. The delocalisation energy or resonance energy makes benzene much more stable than a hypothetical cyclohexatriene molecule with localised carbon carbon double bonds
Simple electrophilic addition to the benzene ring would destroy this system of delocalised pi (π) electrons and as a result benzene and other aromatic molecules will not readily undergo electrophilic addition reactions. Electrophilic addition would involve:
The mechanism for electrophilic addition of a molecule of hydrogen bromide to benzene is outlined below, you can see that in the final product the additional stability of benzene or aromaticity as it is often referred to as due to the presence of the pi (π) delocalised electrons is no longer present:


Or using the circle notation to represent the aromatic ring we have:

 The mechanism for electrophilic substitution reactions can be thought of as occurring in a number of simple steps:
The mechanism for electrophilic substitution reactions can be thought of as occurring in a number of simple steps:
Match the terms with the definitions by simply clicking on the term and its correct definition. Correct responses will turn green.
 The intermediate carbocation formed during an electrophilic substitution reaction will be a resonance stabilised ion.
Addition of the electrophile
to the benzene ring is likely to be the slow step in the above reaction since it will remove
or destroy the aromatic stabilisation
that results from the delocalisation of the pi (π) electrons in the
ring.
The intermediate carbocation formed during an electrophilic substitution reaction will be a resonance stabilised ion.
Addition of the electrophile
to the benzene ring is likely to be the slow step in the above reaction since it will remove
or destroy the aromatic stabilisation
that results from the delocalisation of the pi (π) electrons in the
ring.
Normally when a covalent bond forms the two electrons involved in the bond are held firmly in place between the two nuclei of the atoms forming the covalent bond, however during resonance the electrons involved in forming the covalent bonds are spread out over multiple atoms, the electrons involved in resonance are usually delocalised pi electrons such as those found in aromatic compounds such as benzene.
It is not correct to say that delocalised electrons are free to move within a molecule, but rather the delocalised electrons are spread out over multiple atoms. The ability of a molecule or ion to form many different resonance structures will provide additional stability to the molecule or ion. In the diagram below it is possible to draw three resonance structures
for the intermediate carbocation. Generally the more
resonance structures you
are able to draw the more stable the molecule or ion is likely to be.

Answer the questions below to quickly review your understanding of some of the key ideas covered above.
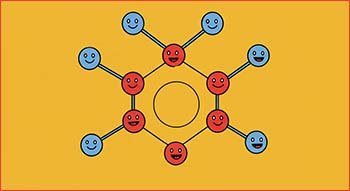
Benzene does not undergo electrophilic addition reactions because simple electrophilic addition would destroy the highly stable delocalised system of pi(π) electrons which would require a large input of energy.
 The intermediate carbocation is resonance stabilised because the electrons involved in forming the covalent bonds between the carbon atoms in the benzene ring are spread out over multiple carbon atoms (delocalised) which allows for multiple resonance structures to be drawn. The more resonance structures that can be drawn the more stable a molecule is
The intermediate carbocation is resonance stabilised because the electrons involved in forming the covalent bonds between the carbon atoms in the benzene ring are spread out over multiple carbon atoms (delocalised) which allows for multiple resonance structures to be drawn. The more resonance structures that can be drawn the more stable a molecule isElectrophilic substitution is a very versatile reaction and it is possible to add a wide variety of substituents onto a benzene ring using this reaction, for example: