The nitration of aromatic rings
is an important reaction industrially since it produces a range
of valuable
compounds which are used mainly in the chemical industry as intermediates in the production of a wide range of industrial products including: explosives, pharmaceuticals, pesticide, plastics
and dyes.

Nitrobenzene is used in the pharmaceutical industry in the synthesis of various drugs, including the analgesic paracetamol but perhaps it most well known use is in the synthesis of the important aromatic amine phenylamine or aniline which is used as a precursor in the production of a vast array of other chemicals including: dyes and pigments, sealants, foams, adhesives, pharmaceuticals and explosives to name but a few of its many uses.
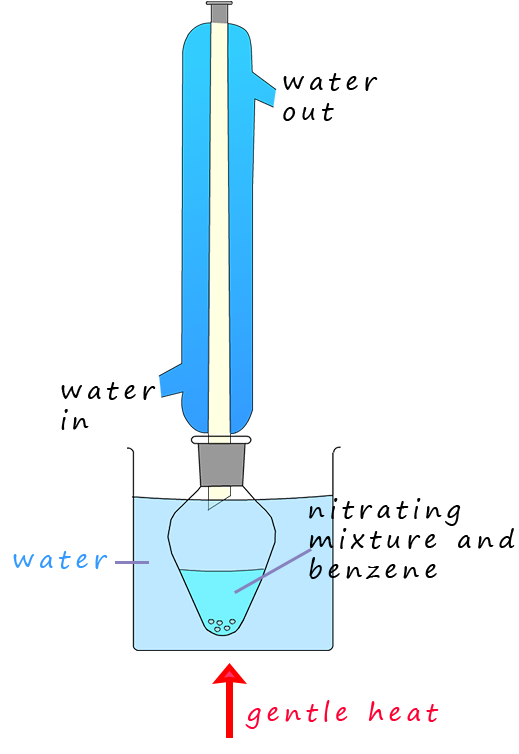
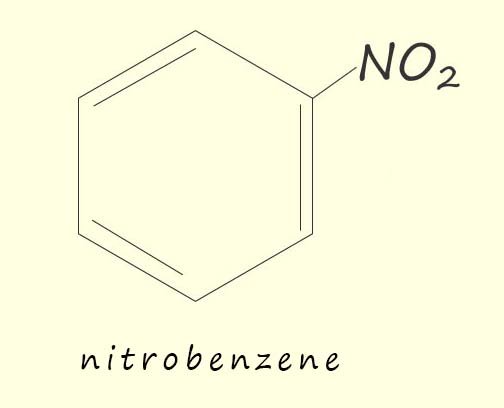
If benzene is warmed under reflux conditions (as shown opposite) to between 50-550C with a 3:1 mixture of concentrated sulfuric acid and nitric acids the main product of this reaction is a yellow oily liquid, nitrobenzene. If the temperature is allowed to rise above about 550C then it is likely that more than one nitro group (-NO2) will be added to the aromatic ring; as shown in the image below.
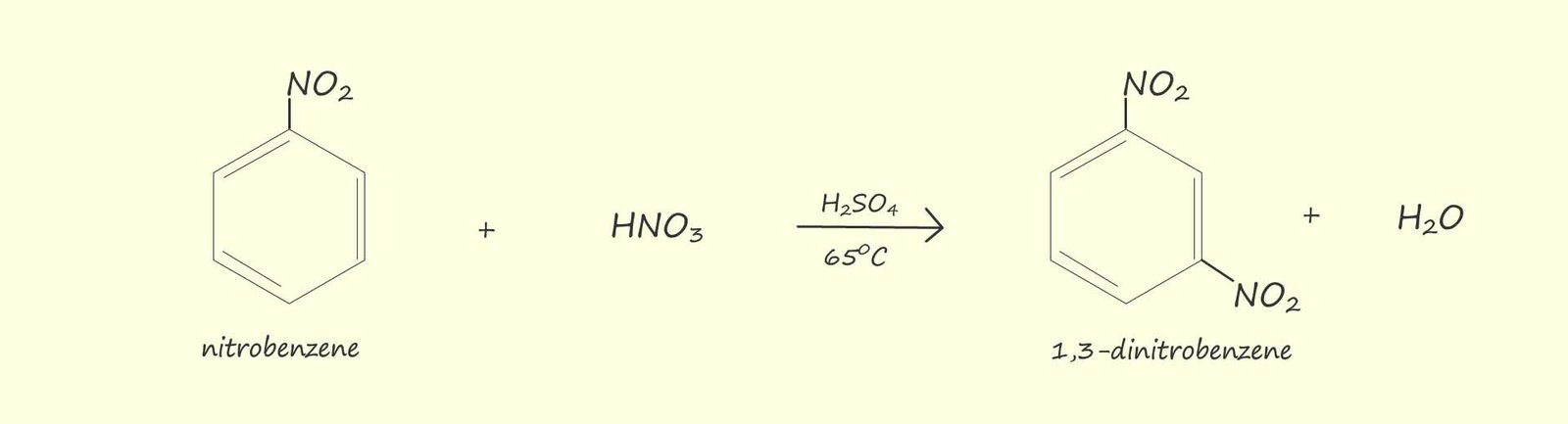
The mixture of
concentrated nitric and
sulfuric acid is reacted with benzene because these two acids react together to produce the
nitronium ion or the nitryl ion (NO2+), which is the
electrophile which will substitute for one of the hydrogen atoms on the benzene ring.
Sulfuric acid (H2SO4) is a stronger acid than nitric acid so when these two acids are mixed the nitric acid essentially acts as a base and is protonated by the sulfuric acid; this acid-base reaction is shown in step 1 of the mechanism for the formation of the nitronium ion (NO2+); shown below. This reaction is reversible and the use of an excess of sulfuric acid will help drive the equilibrium to the right-hand side and produce more protonated nitric acid and hydrogen sulfate (HSO4-) molecules.

In step 2; shown below the unstable intermediate cation formed by the protonation of the nitric acid by the sulfuric acid breaks down to form the nitronium ion (NO2+) and a molecule of water; as shown below.

Now by simply combining the equations above in step 1 and step 2 and cancelling out any species which appear on both the reactants and products side of the equations we can obtain an equation which shows how the nitronium ion (NO2+) is formed:
The equation above shows how the nitronium ion electrophile is generated; however you may see in some textbooks an additional step which includes the role of the concentrated sulfuric acid as a dehydrating agent. Now concentrated sulfuric acid is not only a strong acid it is also a very good dehydrating agent, this means it has a very high affinity for water — it readily reacts with water in a typical acid-base reaction to form the hydronium ion (H3O+) and the hydrogen sulfate ion (HSO4-) according to the equation below, lets call this step 3:
The sulfuric acid will react with the water produced from step 2 and by removing the water from this equilibrium reaction this will help push the position of equilibrium in step 2 to the right hand side and help ensure there is a high concentration of the electrophilic nitronium ion (NO2+); which will obviously help in increasing the yield of the nitrobenzene.
So we can write an overall equation to show how the nitronium ion (NO2+) is formed from the acid-base reaction of concentrated sulfuric and nitric acids, it is simply obtained by combing the equations from steps 1, 2 and 3 and cancelling out any of the same species that appear on the reactants and products sides of the equations:
Now that we have looked at in detail how the nitronium ion; the electrophile is generated lets look at the reaction of the nitronium ion with benzene. This reaction proceeds as you might expect any electrophilic substitution reaction to proceed. The delocalised pi (π) electrons in the benzene ring will now attack the nitronium ion (NO2+) and form a resonance stabilised intermediate arenium carbocation, an arenium ion is simply the intermediate carbocation formed when an electrophile attacks an aromatic ring. This is likely to be the slow step in the nitration reaction since it involves disrupting the delocalised pi (π) electrons in the aromatic ring.

After the addition of the nitronium ion (NO2+) the aromaticity; that is the delocalisation stability offered by the presence of the delocalised pi electrons will be destroyed, however the loss of a proton (H+) in the mechanism shown above will quickly restore the aromaticity within the benzene ring and regenerate the sulfuric acid. In the mechanism shown above the hydrogen ion (H+) is removed from the unstable intermediate arenium carbocation by the hydrogen sulfate ion (HS04-) but it is also possible for any other base such as water which is present to remove this hydrogen ion (H+).
Removal of the hydrogen ion (H+) by the hydrogen sulfate ion will regenerate the sulfuric acid; which is often described as a being a catalyst in the nitration of aromatic compounds.
You can of course use the circle notation instead of the Kekulé notation to represent this electrophilic substitution reaction; the choice is yours as both representations of the aromatic ring are correct.
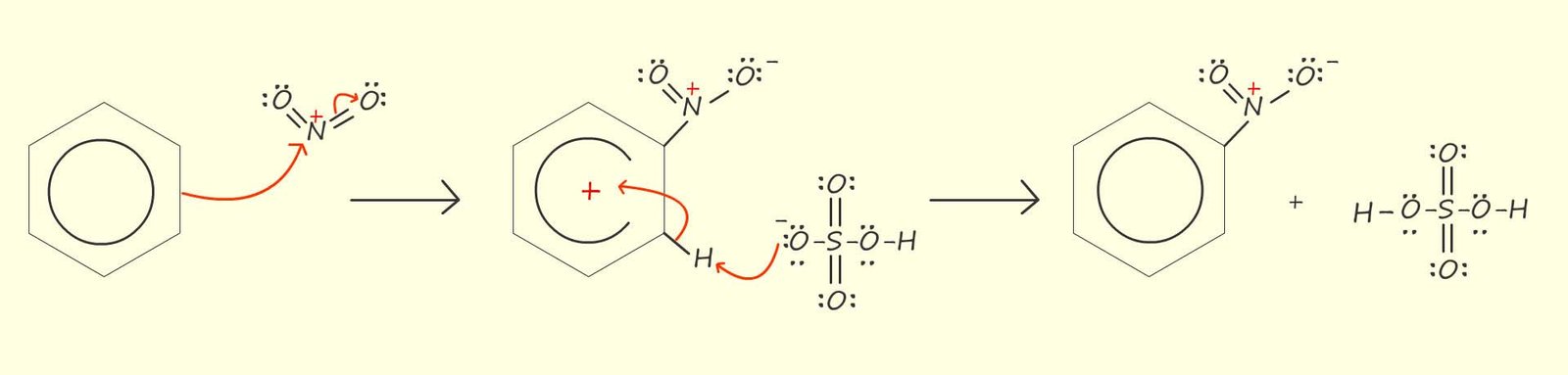
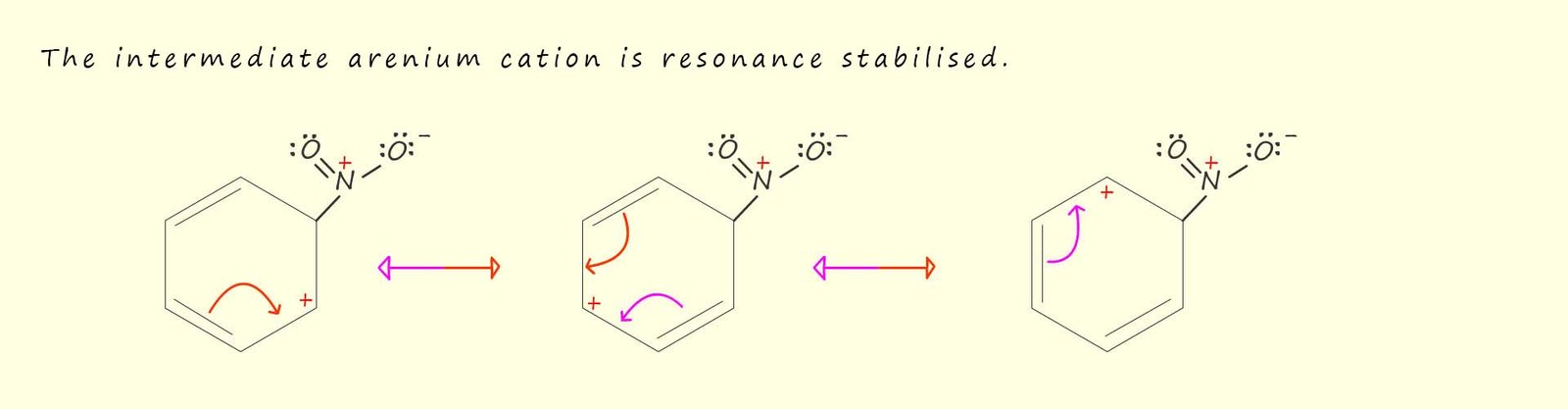
In the mechanism above when the nitronium ion (NO2+) adds to the benzene ring the stability associated with the delocalisation of the pi(π) electrons is lost. In order to help stabilise this intermediate arenium cation it will undergo resonance stabilisation, as shown in the image below. The red portion of the resonance double headed arrow representations the movement of electrons as you travel from left to right across the page while the purple coloured portion of the arrow represents the movement of electrons as you travel from right to left across the page.
Use the flashcards below to quickly review your understanding of the main points mentioned above:

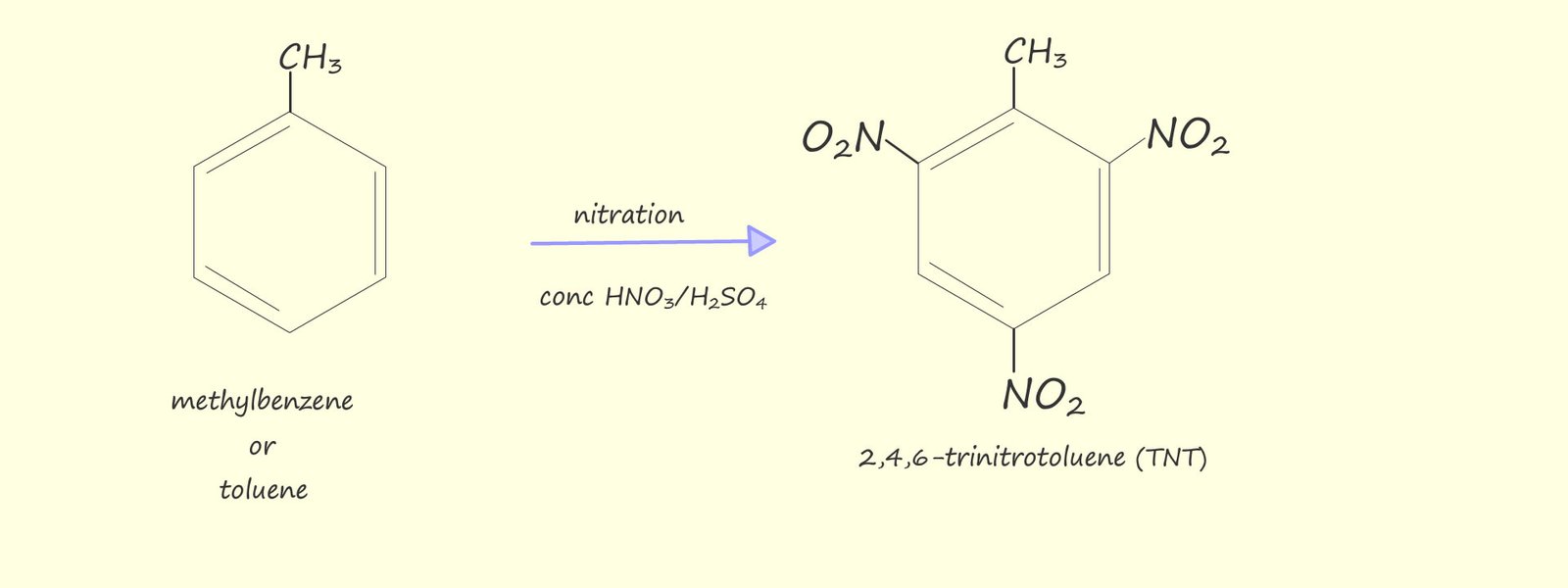
Polynitrated aromatic compounds are used extensively as high explosives; for example: