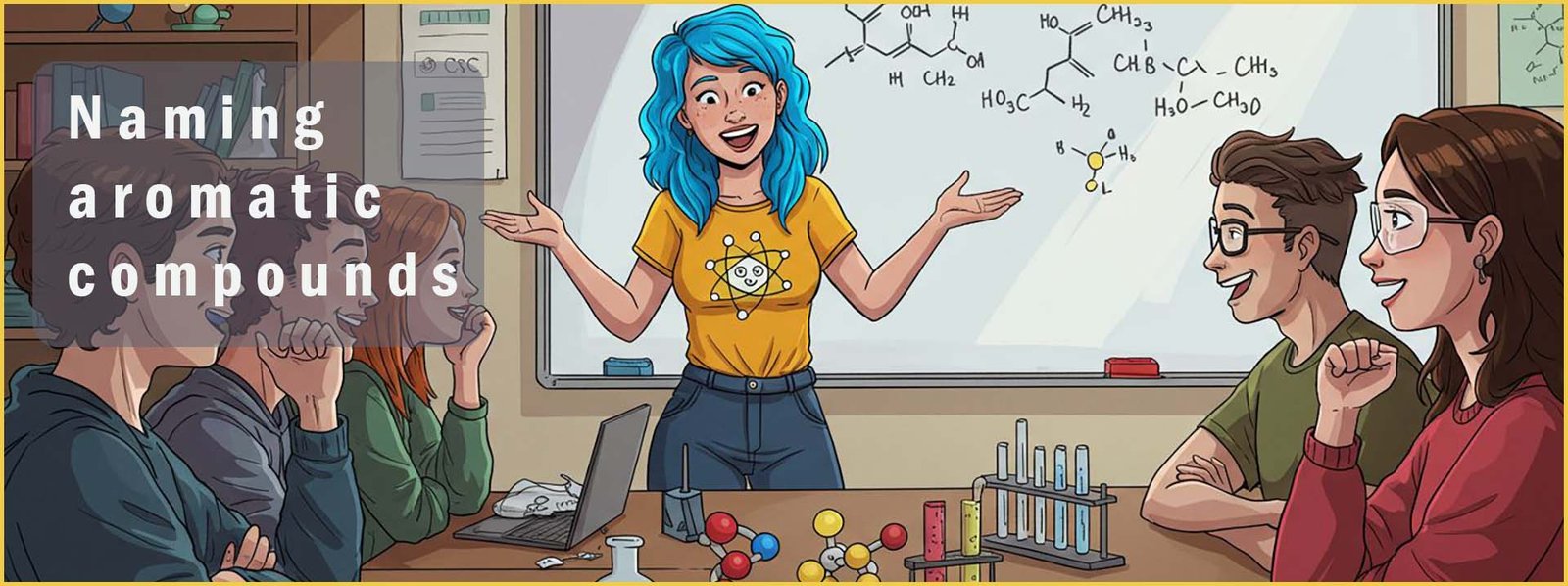

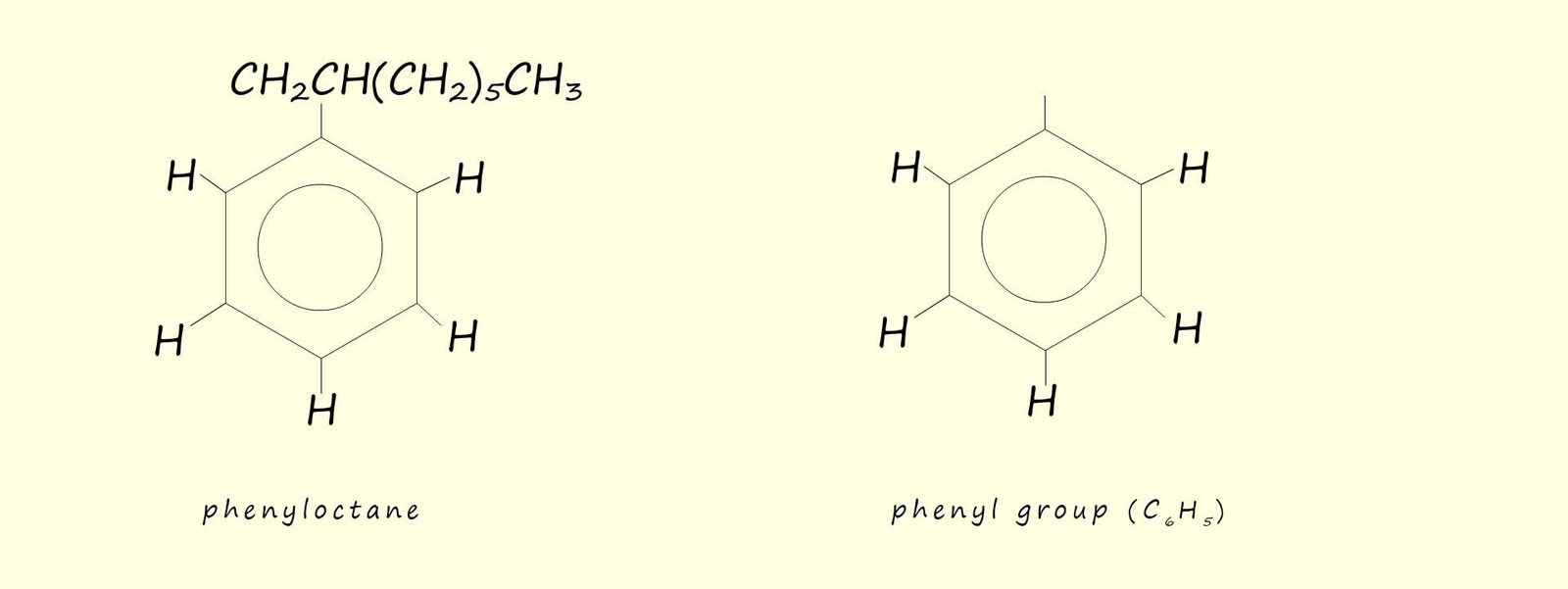
Aromatic compounds will likely contain one or more benzene rings. Benzene has the molecular formula C6H6 with each of the carbon atoms in the planar hexagonal ring covalently bonded to one hydrogen atom. A substituent you will likely meet in your study of organic chemistry is the phenyl group (-C6H5), a phenyl group is simply a benzene ring where one of the hydrogen atoms has been removed. This is shown below:
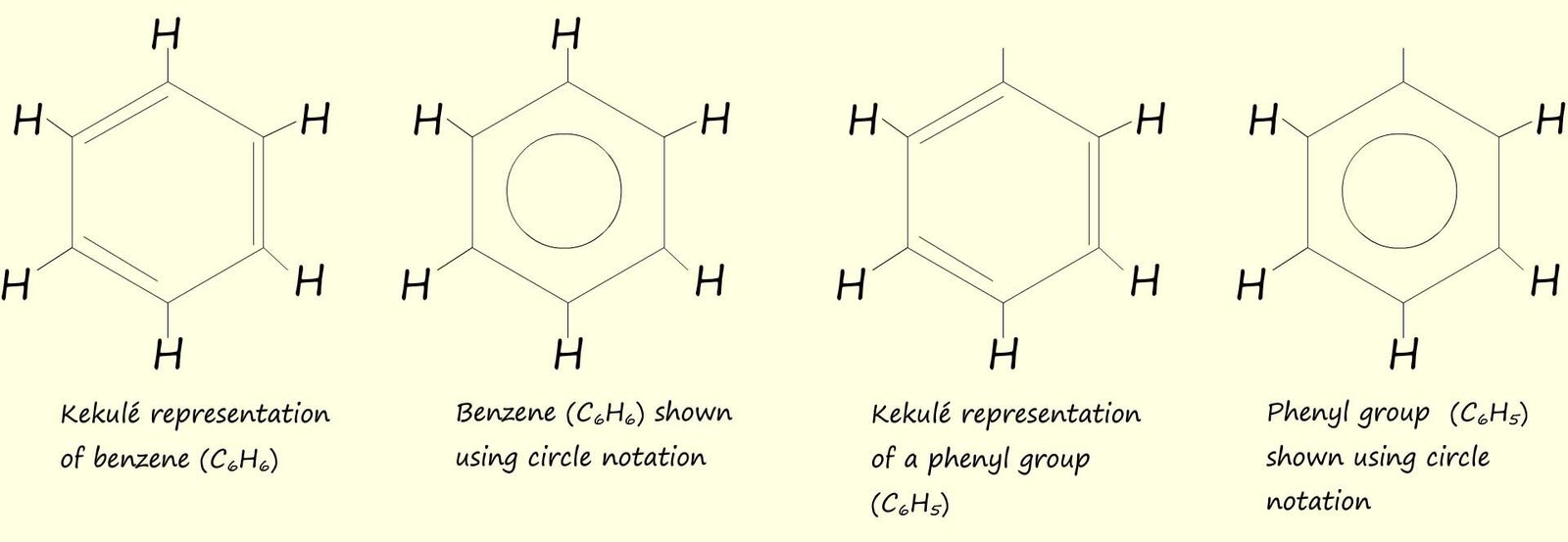
You will no doubt meet aromatic rings which have been substituted with various atoms and groups including: chlorine (-Cl), bromine (-Br), nitro group (-NO2), methyl group (-CH3) as well as many other atoms and groups. Naming these monosubstituted aromatic rings follows the same rules you have used for other hydrocarbon molecules. These molecules are simply named as substituted benzene rings e.g.
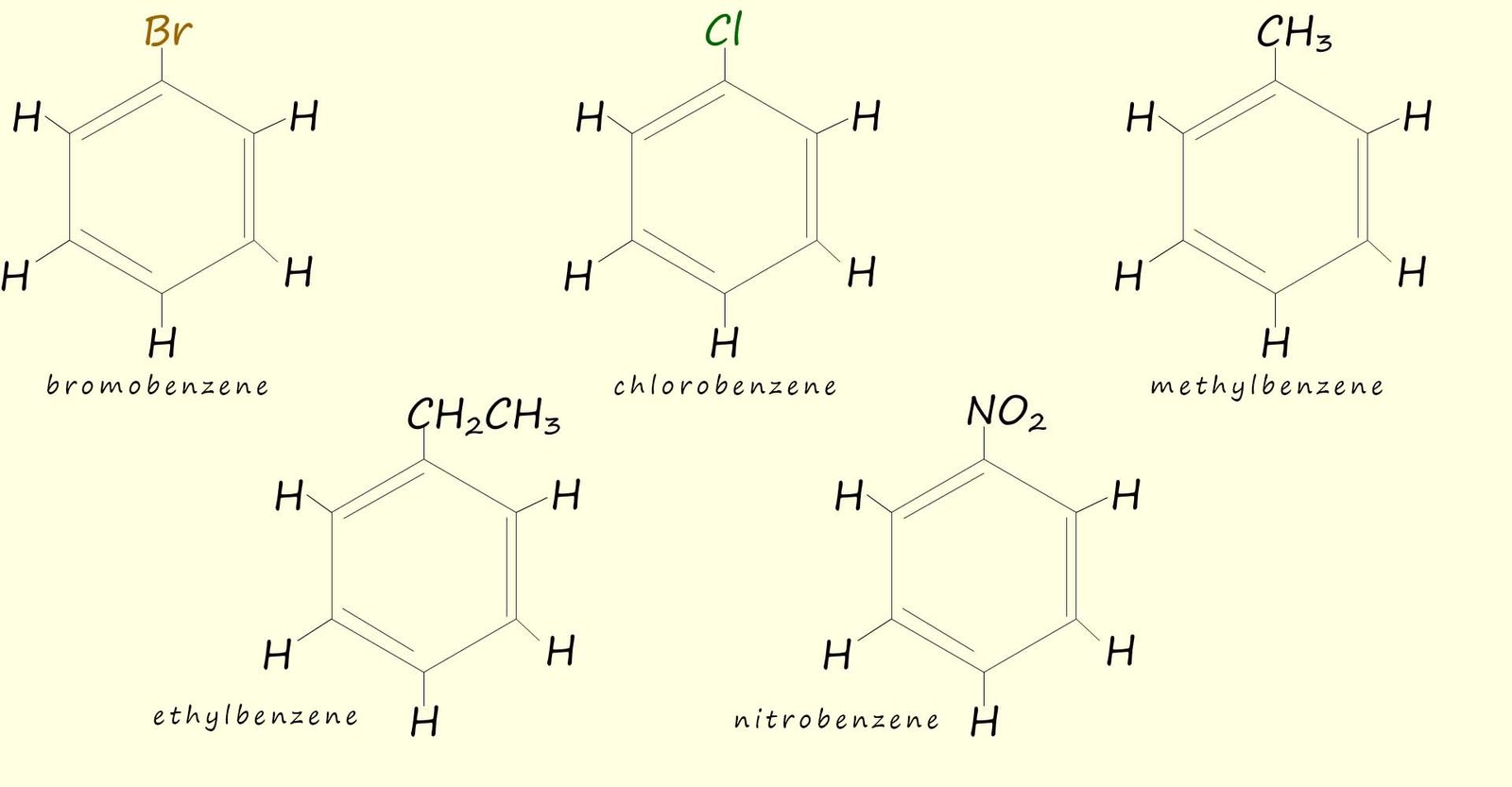
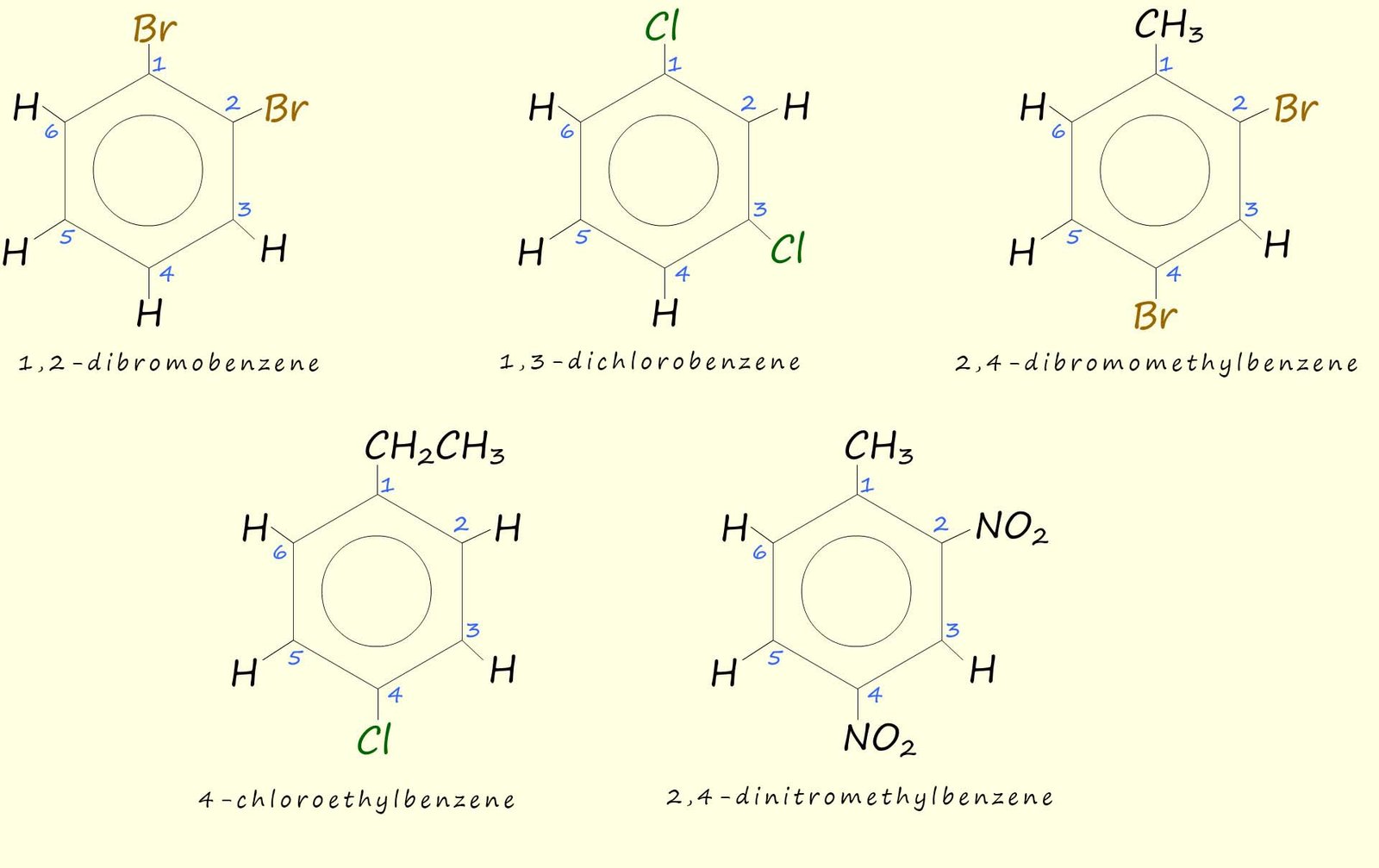
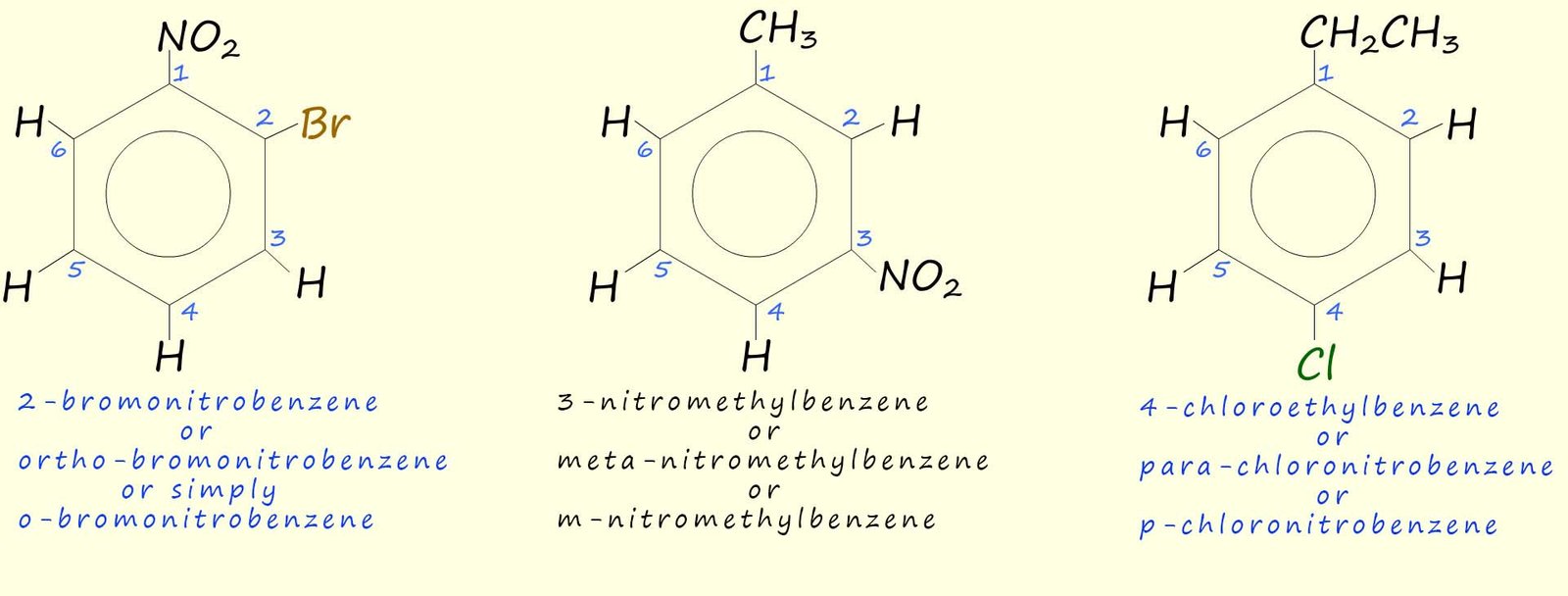
Aromatic rings with more than one attached substituent need to be named by numbering the carbon atoms that any substituents are attached to. The substituents are listed alphabetically and they must be given the lowest possible numbers e.g. consider the following substituted aromatic rings:
Disubstituted benzene rings may also be named using one of the prefixes ortho, meta or para. These three positions are shown below:
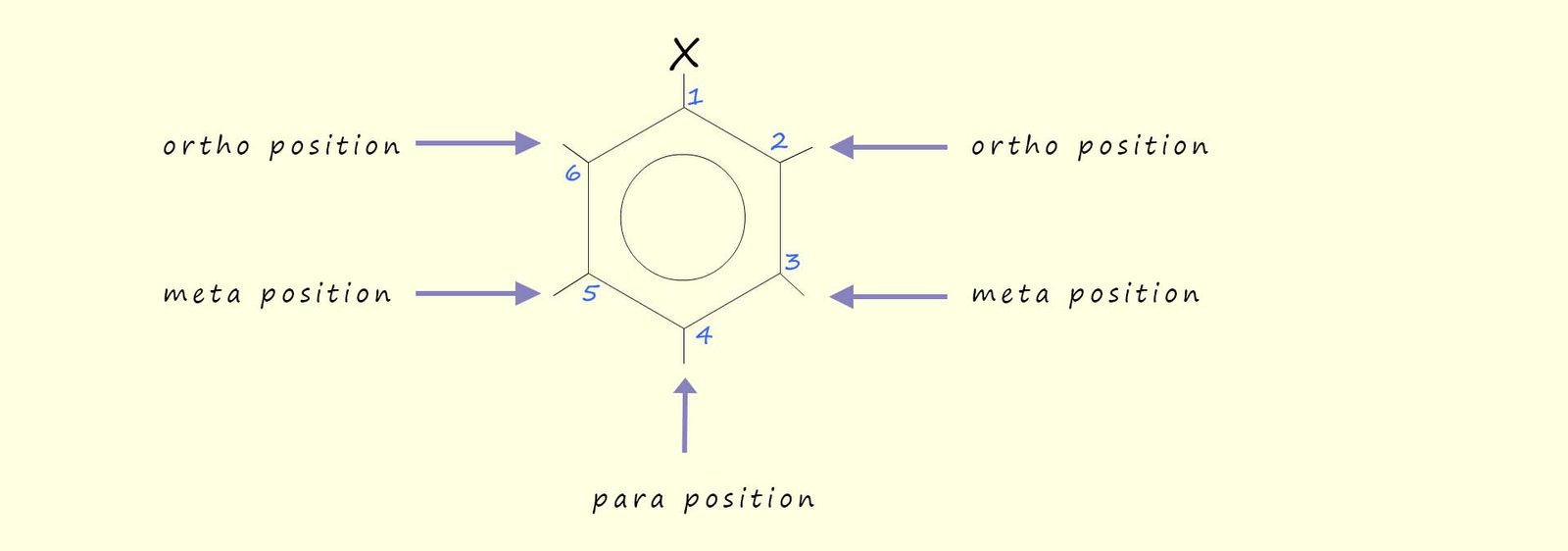
As an example consider the following three molecules:
Alkyl substituted benzene ring are often referred to as arenes. Arenes are named in two different ways, depending on the size of the alkyl substituent:

The naming of aromatic compounds can at times become rather frustrating to new students simply because many traditional trivial names that is non-systematic names are frequently used. Some of these are shown below and I am afraid to say that it is just a case of remembering these names and molecular structures:
