

Note: Before reading this page, make sure you are familiar with nucleophilic substitution reactions involving halogenalkanes. If you need a quick recap, click the links above.
Alcohols are formed when halogenalkanes are warmed with a warm aqueous solution of sodium hydroxide or potassium hydroxide. Here the hydroxide ion (OH-) is acting as a nucleophile and attacking the δ+ carbon in the polar C-X bond present in the halogenalkane molecule 2-bromopropane to form the secondary alcohol propan-2-ol. This is outlined in the image below:
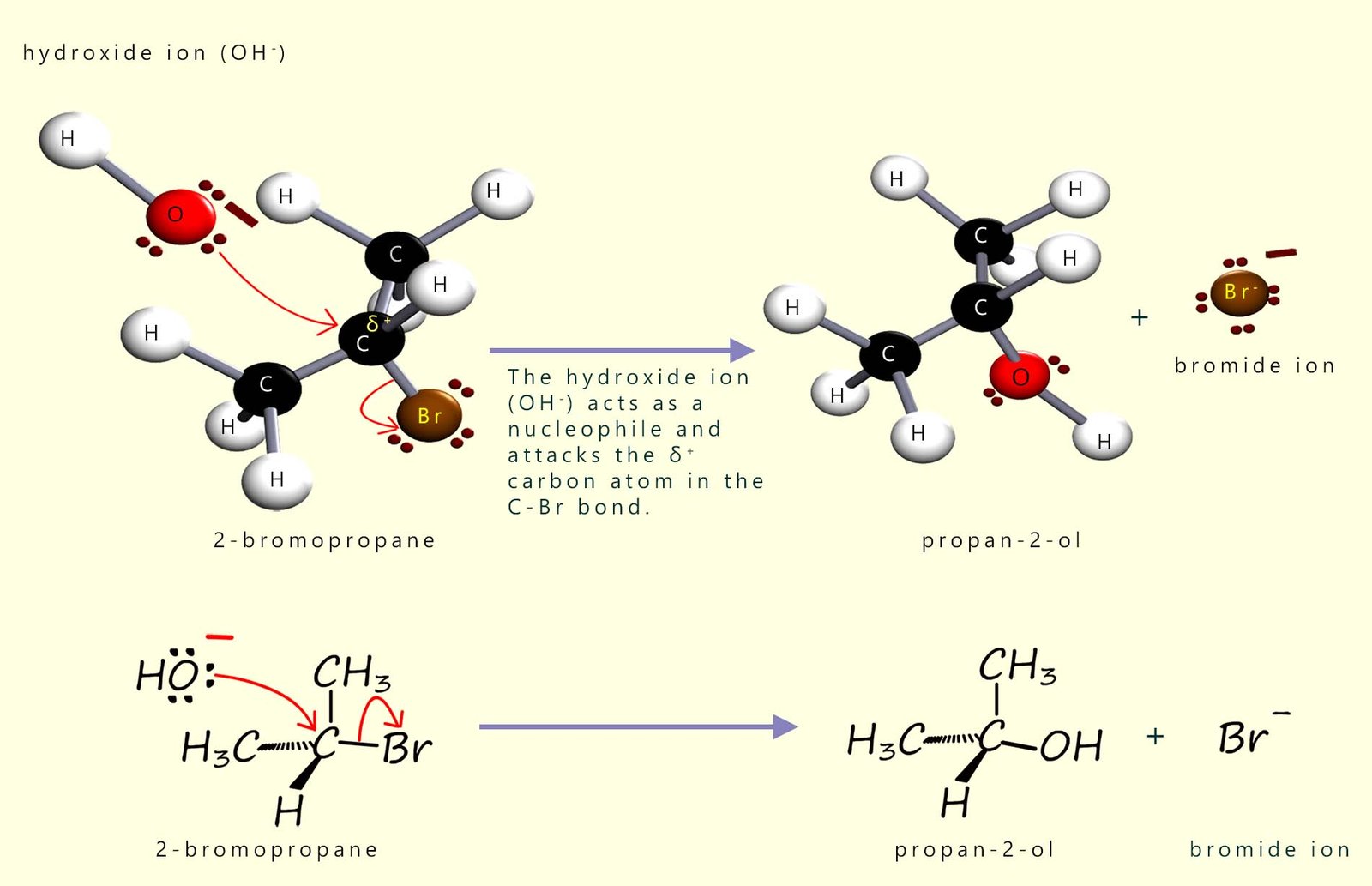
You are probably most familiar with sodium hydroxide and potassium hydroxide from the work you will have done on acids and alkalis, where they act as Brønsted–Lowry (H+ acceptors). However the hydroxide ion (OH-) is not only base but it can also behave as a nucleophile. There are lots of similarities between nucleophiles and bases, for example they both have lone pairs of electrons for a start. Strong bases are often good nucleophiles too, but whether the hydroxide ion will act as a base or a nucleophile depends a lot on the reaction conditions. With hydroxide ions you can push the reaction towards acting mainly as a base or mainly as a nucleophile by changing the solvent and the temperature at which the reaction takes place.
If a halogenalkane is gently warmed with sodium hydroxide or potassium hydroxide dissolved in water (so an aqueous solution), the hydroxide ion behaves as a nucleophile. This leads to a nucleophilic substitution reaction, producing an alcohol.
However by changing the reaction conditions we can alter the reaction that takes place, for example if potassium hydroxide or sodium hydroxide is dissolved in a hot ethanol (an alcoholic solution), then a different type of reaction will take place, this time rather than a nucleophilic substitution reaction occurring an elimination reaction will take place. Under these hot alcoholic conditions the hydroxide ion (OH-) acts mainly as a base (H+ acceptor) rather than as a nucleophile. At A-level this is usually described as a one-step elimination reaction. Key features of a typical elimination reaction you should know are:
This is shown below:
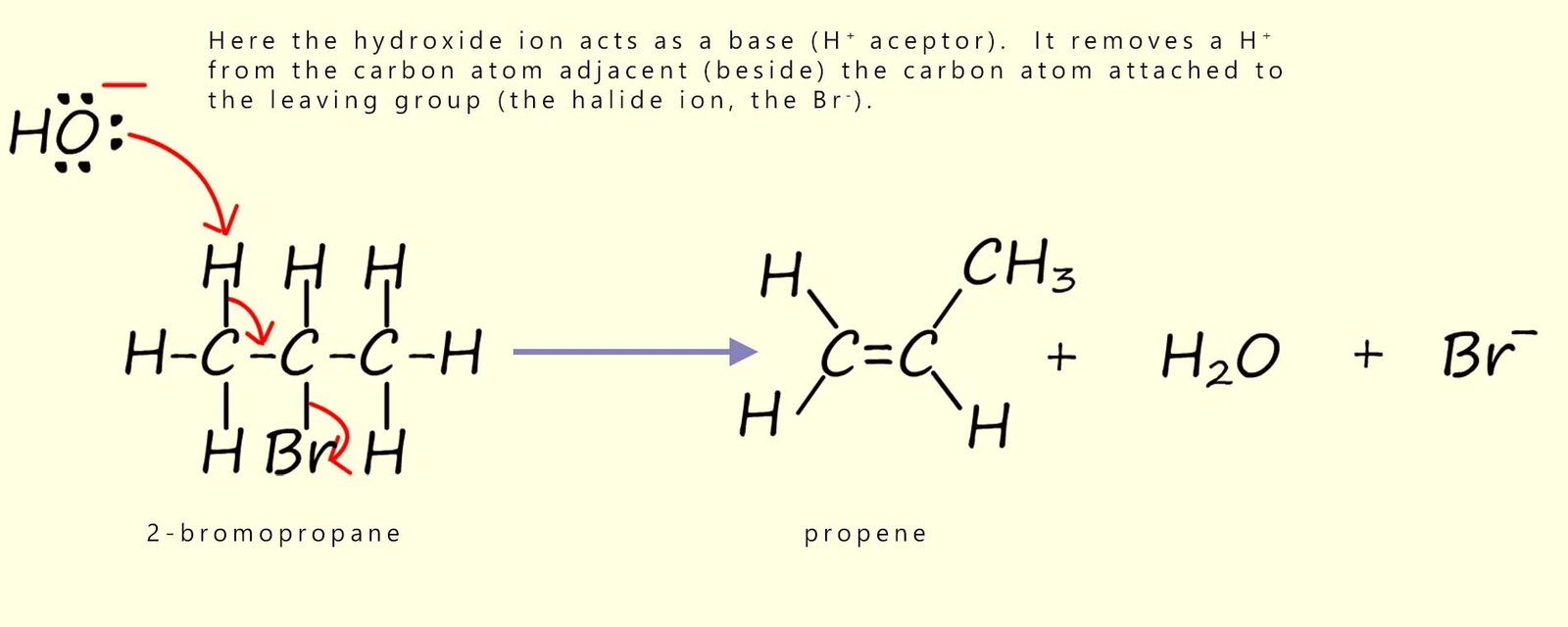
In base-induced elimination reactions of haloalkanes (for example with ethanolic KOH), more than one alkene can sometimes form.
Zaitsev’s rule helps you predict which one is formed most 👇
📝 Exam tip: Zaitsev’s rule works best for secondary and tertiary haloalkanes.
In the example above using 2-bromopropane (a
symmetrical halogenalkane),
it would not make any difference which adjacent carbon you remove a hydrogen from, because you still end up with alkene, propene in this case.
However if you use an unsymmetrical
halogenalkane then more than one alkene can be formed.
As an example consider the products formed from heating
2-bromobutane (an unsymmetrical secondary haloalkane) with
hot alcoholic potassium hydroxide.
Predicting the exact products of elimination reactions can initially be a little trickier than those formed during nucleophilic substitution reactions. However to find all possible products that can be formed simply locate the halogen atom in the haloalkane molecule and remove one hydrogen atom from each of the carbon atoms adjacent to the one bonded to the halogen. Now elimination reactions often results in a mixture of alkenes.
For 2-bromobutane, the base (OH-) can remove hydrogen atoms on either adjacent carbon bonded to the halogen; as outlined in the mechanism below; so two different alkene molecules can be formed, now one of the alkene molecules formed can exist as a pair of geometric isomers- this "little trick" is a very common exam question; as many students fail to recognise the possibility of geometric isomerism occurring. This is shown in the mechanism below:
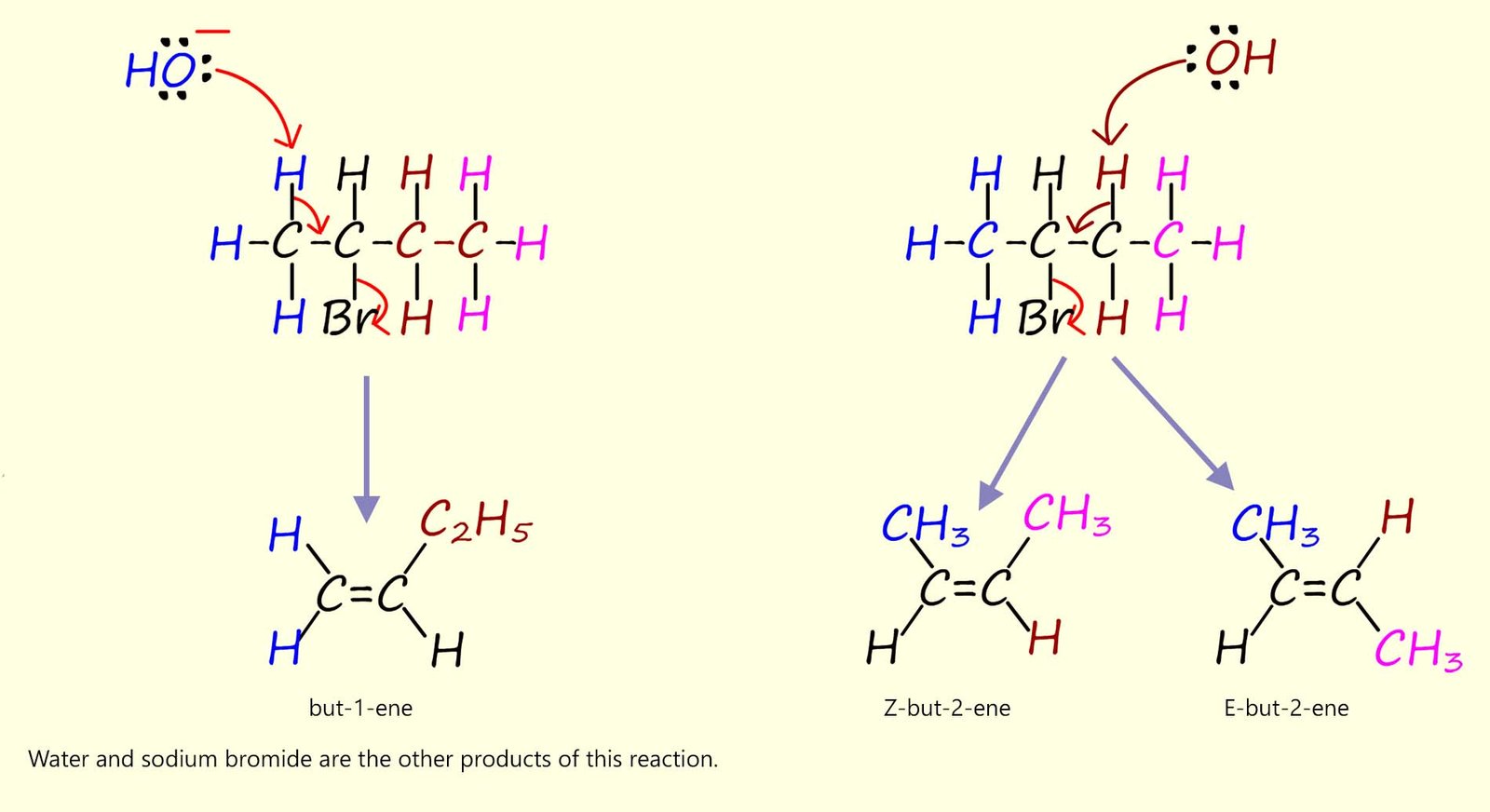
Even though a mixture of products is formed they are not produced in equal amounts during these base-induced elimination reactions. Now we can use Zaitsev's rule (see the brain box above) to help in predicting which product will be the major one produced and which product is produced in smaller amounts. According to Zaitsev's rule the
more substituted alkene; that is the one with the least number of hydrogen atoms bonded to the C=C bond is usually the
major product.
In the example above but-2-ene is more substituted than but-1-ene so it will be the main product of this reaction.
However nucleophilic substitution reactions can compete with elimination reactions; especially with secondary
halogenalkanes and so you may get some alcohol products as well.
In general elimination reactions are favoured most strongly with tertiary
halogenalkanes; while primary
halogenalkanes are more likely to give substitution products; that is alcohols.
Note: Primary halogenalkanes can still undergo elimination reactions under hot ethanolic conditions, but nucleophilic substitution is often a big competitor here.
Reaction conditions also help decide whether you get mainly nucleophilic substitution or mainly elimination reactions:
Why not use the summary table below to create some flashcards to help you recall the reaction conditions and the products formed!
| What you’re doing 🧪 | Conditions 🔥 | What OH- acts as 👇 | Main product ✅ | Quick clue 💡 |
|---|---|---|---|---|
| Nucleophilic substitution |
Warm aqueous NaOH / KOH (OH- in water) |
Nucleophile attacks the δ+ carbon |
Alcohol 🧴 | Water solvent → substitution is favoured |
| Elimination |
Hot ethanolic NaOH / KOH (OH- in ethanol) |
Base removes H+ from adjacent carbon |
Alkene (C=C) ⚡ | Hot + ethanol → elimination is favoured |
| Elimination (unsymmetrical haloalkane) | Same: hot ethanolic base | Base |
Mixture of alkenes 🧩 often one is major |
Use Zaitsev’s rule: more substituted alkene is usually the major product ⭐ |
Click the button below to open the quiz.
For each situation below, decide whether the reaction is more likely to be nucleophilic substitution or elimination.
1) A primary halogenalkane is warmed with aqueous sodium hydroxide.
2) A secondary halogenalkane is heated with hot ethanolic potassium hydroxide.
3) A tertiary halogenalkane reacts with hot ethanolic hydroxide ions.
4) A halogenalkane reacts slowly in a warm aqueous solution of hydroxide ions.
5) Which condition most strongly favours elimination?
You see: KOH / NaOH + a
halogenoalkane and the question asks for the product…
Do you instantly write an alcohol or an alkene? 😬
📝 Quick check: if the solvent isn’t stated, don’t assume — the examiner may be testing this exact trap.