

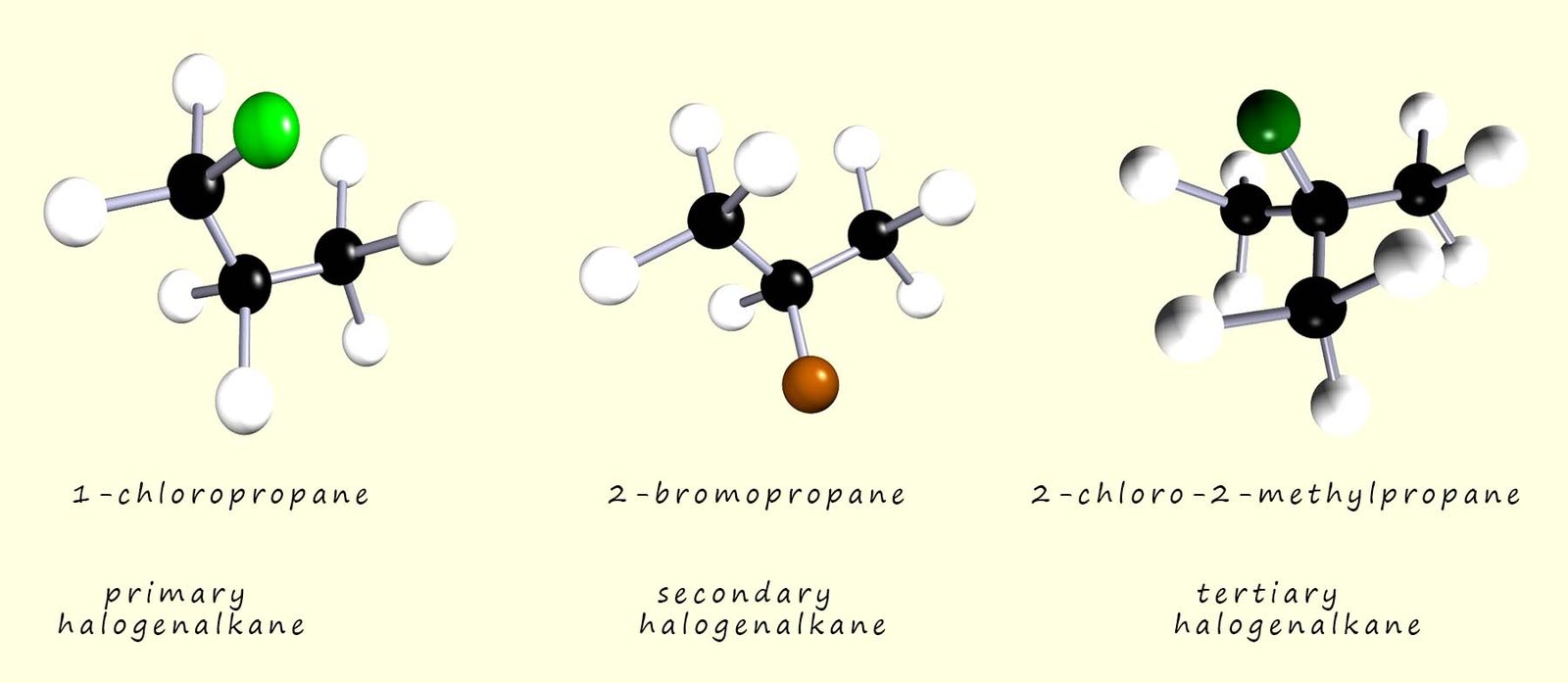
The halogenalkanes or haloalkanes form a homologous series of compounds with the general formula CnH2n+1X, where X is one of the halogens (F, Cl, Br or I). Some simple examples of primary, secondary and tertiary halogenalkane molecules are shown in the image below:
The halogens are generally electronegative
elements, which means that the C-X bond in the halogenalkane molecules
will be a polar one with
the carbon atom in the C-X bond having a partial positive charge (δ+)
and the halogen atom having a
partial negative charge (δ-).
A nucleophile is an electron rich species; nucleophiles can be neutral molecules or ions with lone pairs of electrons which they can donate to an electron deficient molecule/atom, that is an electrophile. Examples of nucleophiles include hydroxide ions (OH-), cyanide ions (CN-), ammonia (NH3) and water (H2O). All these molecules whether charged or neutral are able to act as nucleophiles simply because they have lone pairs of electrons.
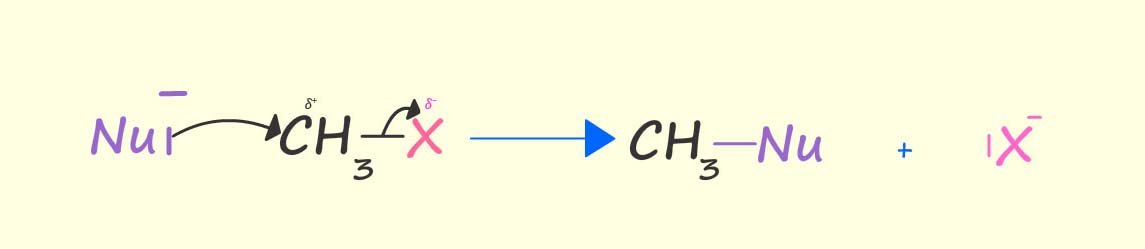
The electron deficient carbon atom attached to the halogen in a halogenalkane molecule is susceptible to attack by electron rich species; that is nucleophiles with lone pairs of electrons. The image below shows how a nucleophile can use its lone pair of electrons to remove and ultimately take the place of the halogen in a halogenalkane molecule; that is the nucleophile substitutes for the halogen atom in the halogenalkane molecule. This is an example of a nucleophilic substitution reaction.
For more details on the mechanisms of these nucleophilic substitution reactions visit the page on SN1 and SN2 reactions which describes in detail the type of reactions undergone by primary, secondary and tertiary halogenalkane molecules.
It is possible to prepare alcohols by hydrolysing halogenalkanes with water; however the reaction is slow simply because water is not a particularly good nucleophile and the halogenalkanes are barely soluble (or not soluble at all) in water, so an aqueous ethanol mixture is a better choice as a solvent since the halogenalkanes are soluble in it. This increases the rate of the hydrolysis reaction:
The acid produced (HX(aq)) in the above equation will be either hydrochloric acid (HCl), hydrobromic acid (HBr) or hydroiodic acid (HI) depending on
which halogen is present in the halogenalkane used. It is also worth mentioning that the (aq) state symbol used
after the halogenalkane
does not mean it is dissolved in water (since halogenalkanes are largely
insoluble in water); it means the reaction mixture is in solution, typically with an aqueous ethanol solvent.
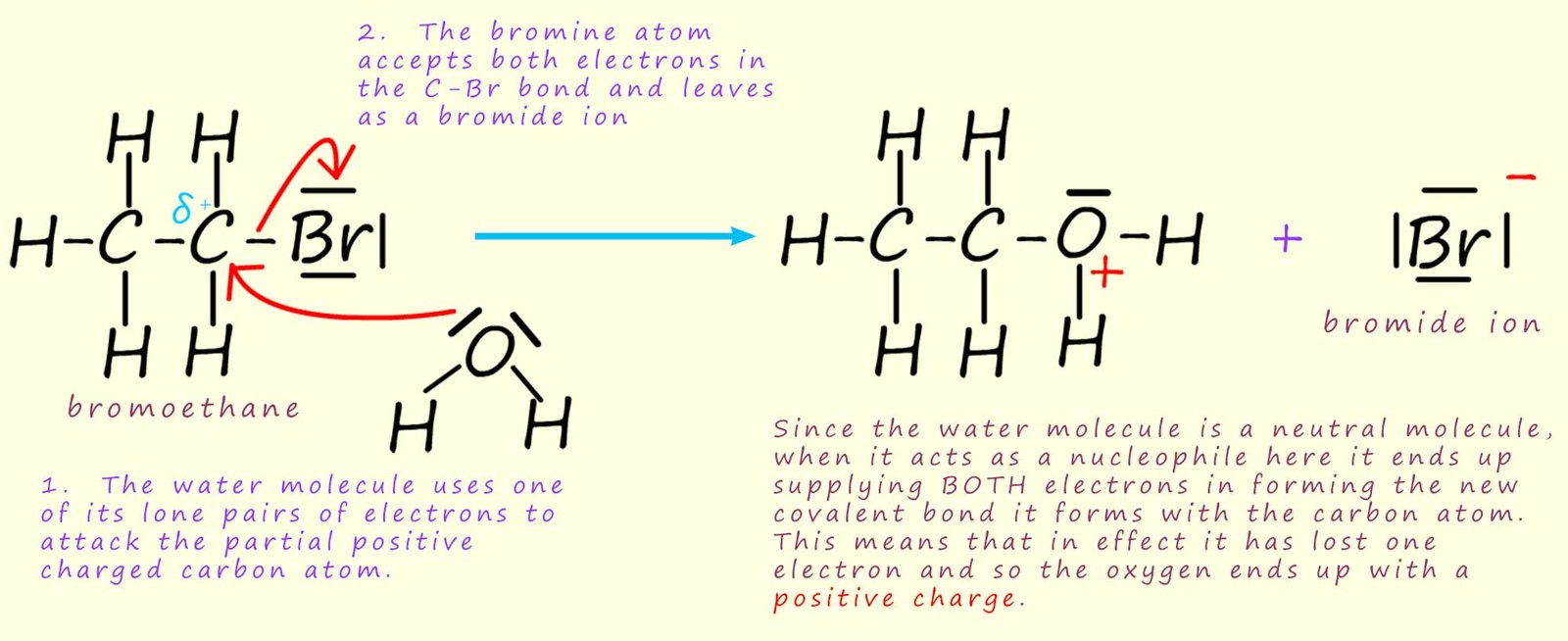
This hydrolysis reaction can be thought of as occurring in two steps. An example of this nucleophilic substitution reaction is shown below using the primary halogenalkane bromoethane as an example:
Here the water molecule uses its lone pair of electrons to act as a nucleophile and attack the partially charged carbon atom (Cδ+). In the example below bromoethane is being hydrolysed with water. An intermediate is formed which contains an oxygen atom with a positive charge - an oxonium ion.
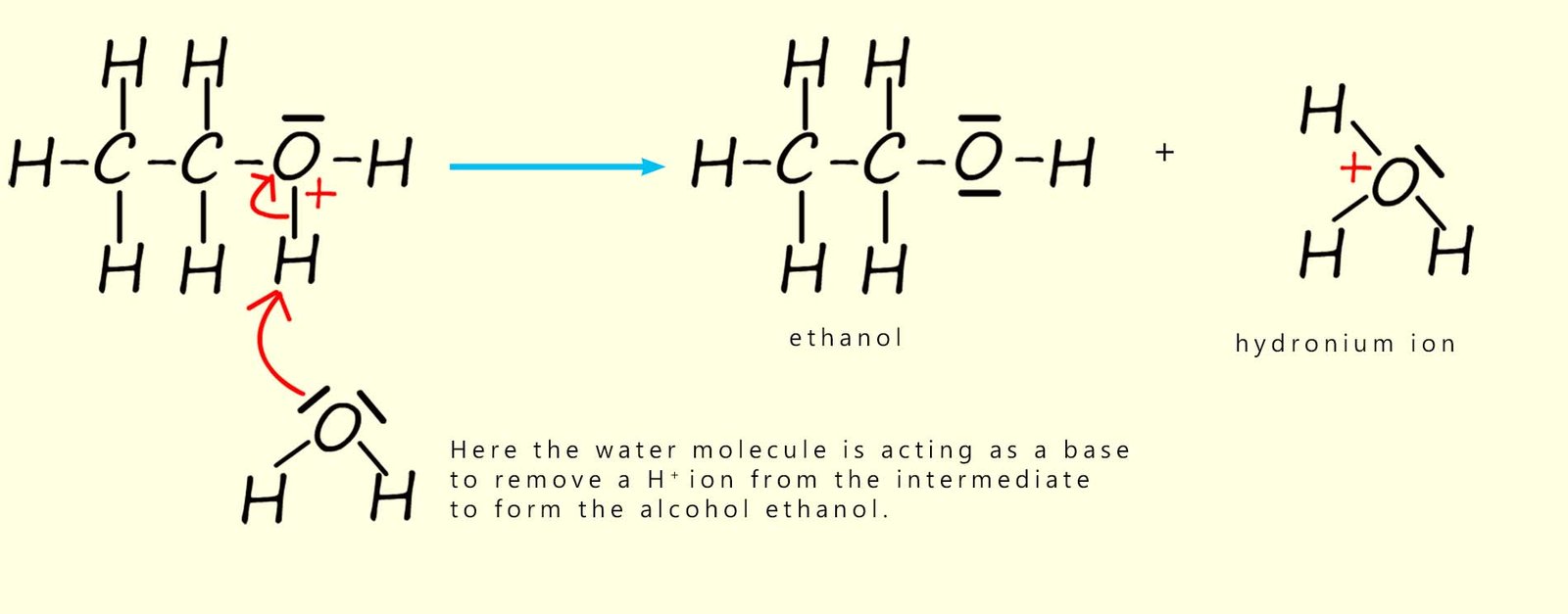
To complete the reaction the intermediate ion formed above has a proton (H+) removed from it by a water molecule. Here the water molecule is acting as a base by accepting a hydrogen ion (H+) to form the alcohol ethanol. The water now forms a hydronium ion (H3O+). This hydronium ion is often simply represented in many equations as H+. This is outlined below.
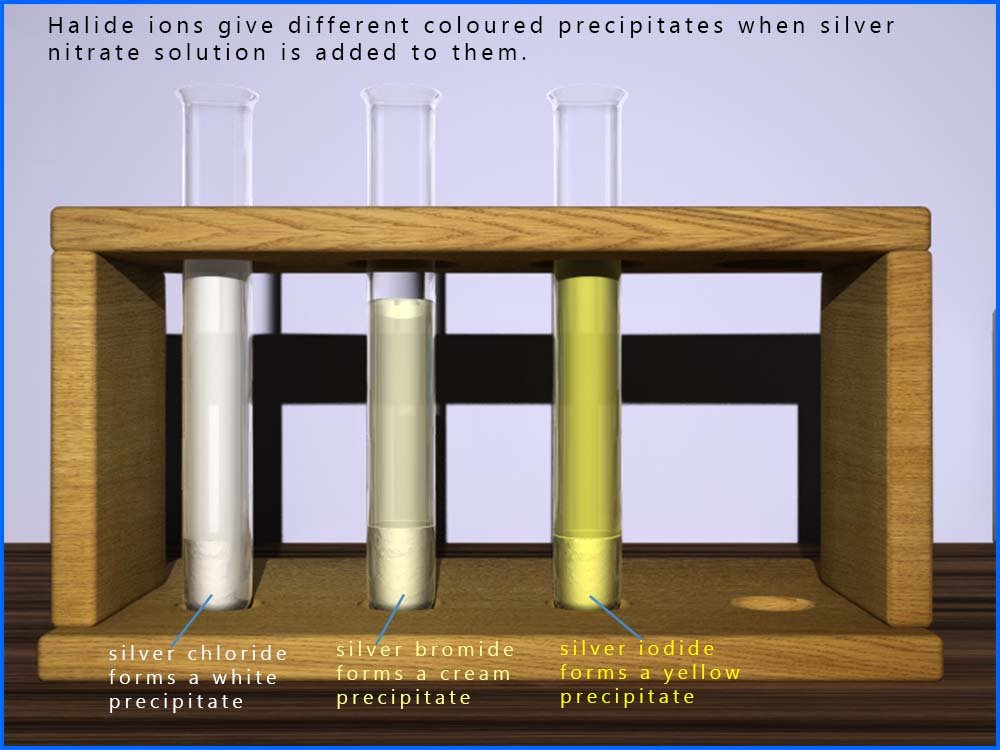
In the hydrolysis reaction of a halogenalkane as shown above we can easily measure the rate of reaction by making use of a reaction you will have seen before, that is the reaction of silver nitrate solution with halide ions (Cl-, Br-, I-) to form white, cream and yellow precipitates of the silver halide. This can be shown as:
For example you may recall that chloride ions (Cl-) will react with a solution of silver nitrate to form a white precipitate of silver chloride.
Similarly bromide ions (Br-) will give a cream coloured precipitate of silver bromide and iodide ions (I-) will form a yellow precipitate of silver iodide.
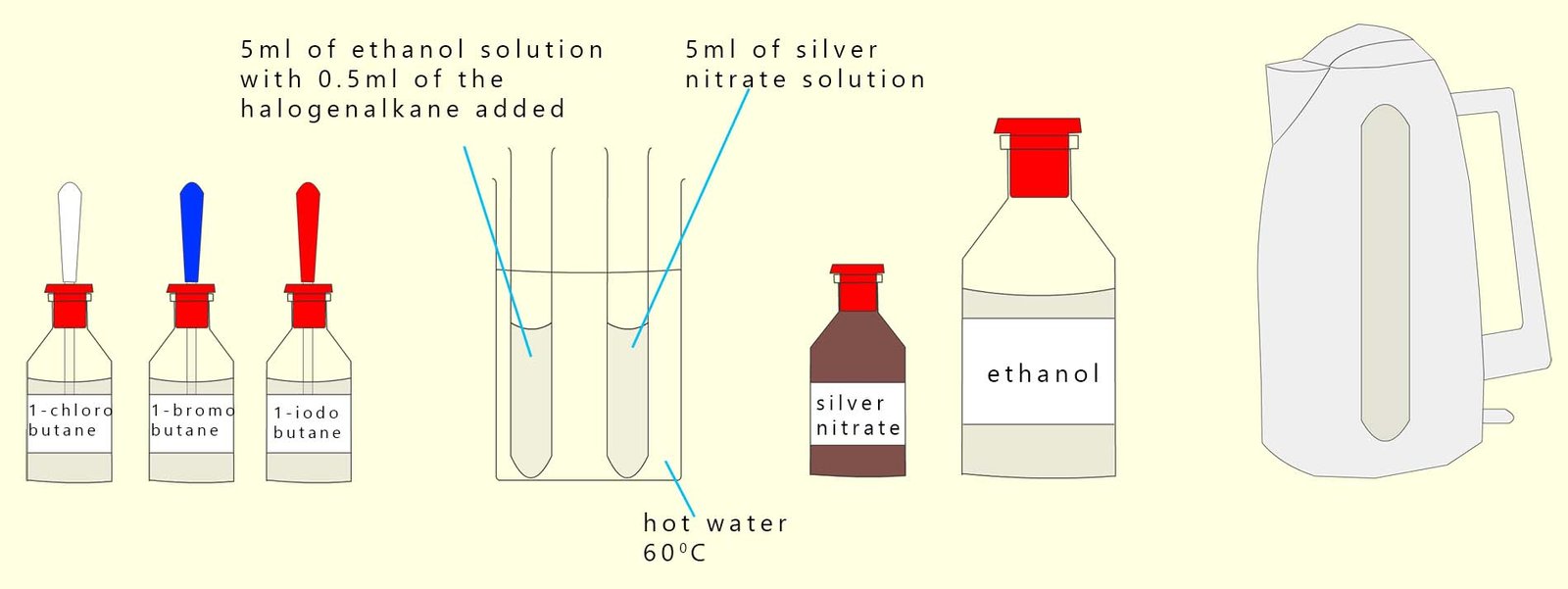
You will also need three other test-tubes; each of these should contain 5 ml of acidified silver nitrate solution. The basic set-up is shown below although I have only shown two test-tubes in the water bath whereas in reality there should be six test-tubes; 3 test-tubes of the acidified silver nitrate and 3 test-tubes containing each of the halogenalkanes dissolved in hot ethanol.
Pour the acidified silver nitrate solution into each of the test-tubes and start the stopwatch. Time how long it takes for each of the coloured white, cream and yellow precipitates of the silver halide to form.
As mentioned above the hydrolysis reaction of halogenalkanes with water is a slow one. However, one of the main factors affecting the rate of this reaction is the bond enthalpy or bond strength of the C–X bond. During this nucleophilic substitution reaction this bond must be broken and the strength of the C-X bond is one of the main factors that determines the rate of this reaction.
The brain box opposite shows the average bond enthalpies of the C–X bonds and we can see that the bond enthalpy decreases as we descend Group 7. Therefore based on this information we can order the rate of reaction as:
In the reaction above we used primary haloalkanes but the halogen present in each case was different. We can also use the above reaction to investigate the rates of hydrolysis of primary, secondary or tertiary halogenalkanes. Here the order of the rate of reaction is:
The reasons for the differences here are in the mechanisms of these reactions and in the conditions used. Though it does not matter if you use primary, secondary or tertiary halogenalkanes, similar trends are seen in the order that iodo react faster than bromo which reacts faster than chloro halogenalkanes.
| 🧠 Idea | ✅ Key point | 📌 Exam link |
|---|---|---|
| What is happening? | Hydrolysis breaks the C–X bond and replaces X with –OH to form an alcohol. | It is a nucleophilic substitution reaction. |
| Why is it slow? | Water is a weak nucleophile and halogenalkanes are poorly soluble in water. | Aqueous ethanol is used as a solvent to help the reaction. |
| Rate trend (Group 7) | As C–X bond enthalpy decreases down the group, hydrolysis gets faster. | iodo > bromo > chloro > fluoro |
| Rate trend (structure) | Tertiary halogenalkanes generally hydrolyse faster than secondary, then primary. | tertiary > secondary > primary |
| How to measure rate | Mix with acidified silver nitrate and time the precipitate forming. | Faster precipitate = faster hydrolysis. |
| Silver halide colours | AgCl is white, AgBr is cream, AgI is yellow. | Shows which halide ion was released. |