

Unsaturated molecules such as alkenes readily undergo electrophilic addition reactions. You may recall from your GCSE science lessons that small molecules can "add" across the carbon-carbon double bond (C=C) in alkenes to form new saturated molecules. The addition of small halogen molecules such as bromine and chlorine along with an explanation of the mechanism for these electrophilic addition reactions were discussed on the page covering simple addition reactions. If you need to quickly refresh your memory on the mechanism of electrophilic addition then click the link before going through the rest of this page.
This page extends the range of electrophilic addition reactions by covering the addition of hydrogen halides to alkenes, including how bond polarity affects how readily these reactions occur in the gas phase, and why the reactivity trend changes in aqueous solutions.
The hydrogen halides are all gases at room temperature; examples of hydrogen halides include hydrogen chloride (HCl), hydrogen bromide (HBr) and hydrogen iodide (HI). All the hydrogen halides have polar bonds, meaning that the atoms in these small molecules have partial charges (δ+ and δ-). The hydrogen atoms in the hydrogen halides all have a δ+ charge, while the halogen atom has a δ- charge. These partial charges arise from differences in the electronegativity values of hydrogen and the halogen atom in the molecule.
However as we move down Group 7 from chlorine to iodine the electronegativity of the halogens decreases. This means that the covalent bond in the hydrogen halide molecules becomes less polar as we move from HCl to HI; as the bond becomes less polar, the size of the dipole moment will also decrease. Recall that the dipole moment is simply a measure of how polar a bond is: the more one atom pulls the bonding electrons towards itself, the larger the partial charges on each atom will be and the larger the dipole moment. This trend is outlined in the image below:
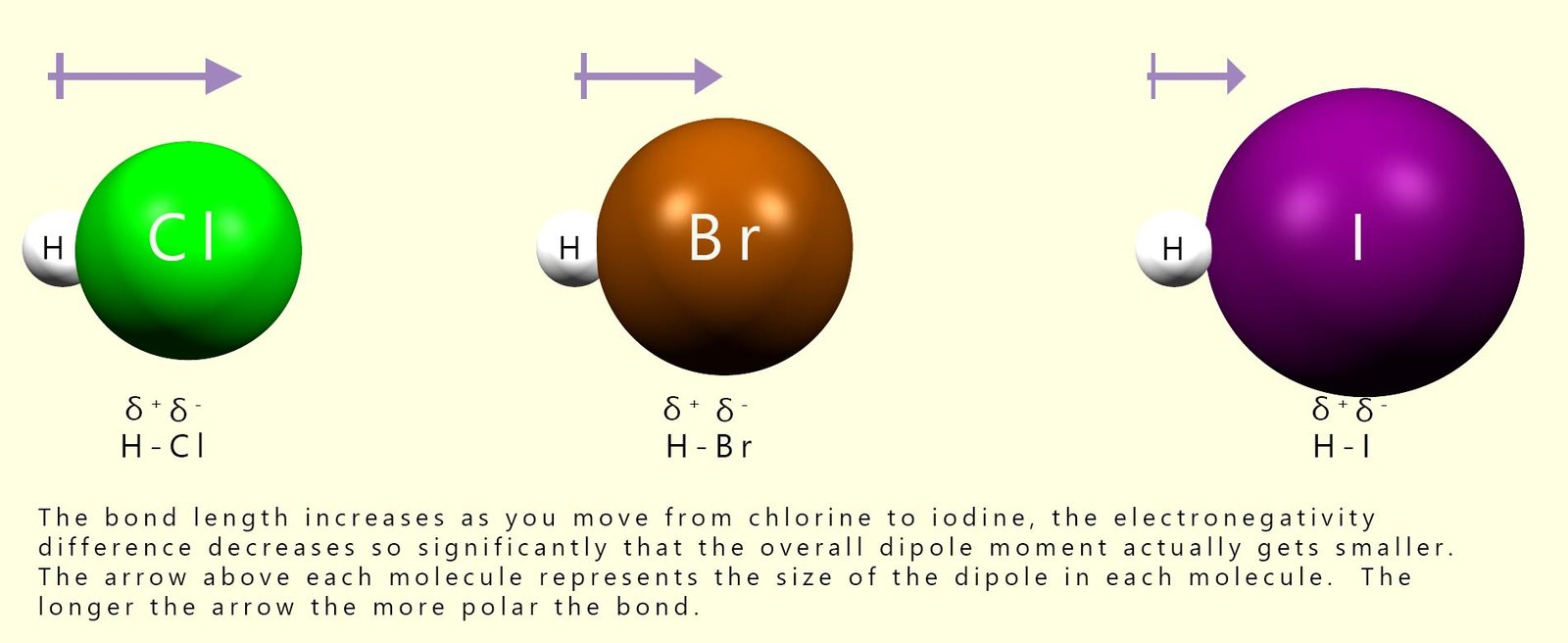
The first step in an electrophilic addition reaction is the attack of the pi (π) electrons in the alkene carbon-carbon double bond (C=C) on an electrophile. In the hydrogen halides the electrophile is the δ+ hydrogen atom. As the size of the dipole decreases as we move from H-Cl to H-I, the hydrogen atom becomes less δ+ and therefore it acts as a weaker electrophile. This clearly affects how readily a particular hydrogen halide will undergo electrophilic addition reactions with alkene molecules. We can summarise this as:
Hydrogen halides with more polar H-X bonds undergo electrophilic addition more readily because the hydrogen atom is more strongly partially positive and acts as a better electrophile.
The mechanism for the gas phase reaction of the alkene ethene with hydrogen bromide gas is outlined in the image below. A similar electrophilic addition reaction will occur with hydrogen chloride and hydrogen iodide.
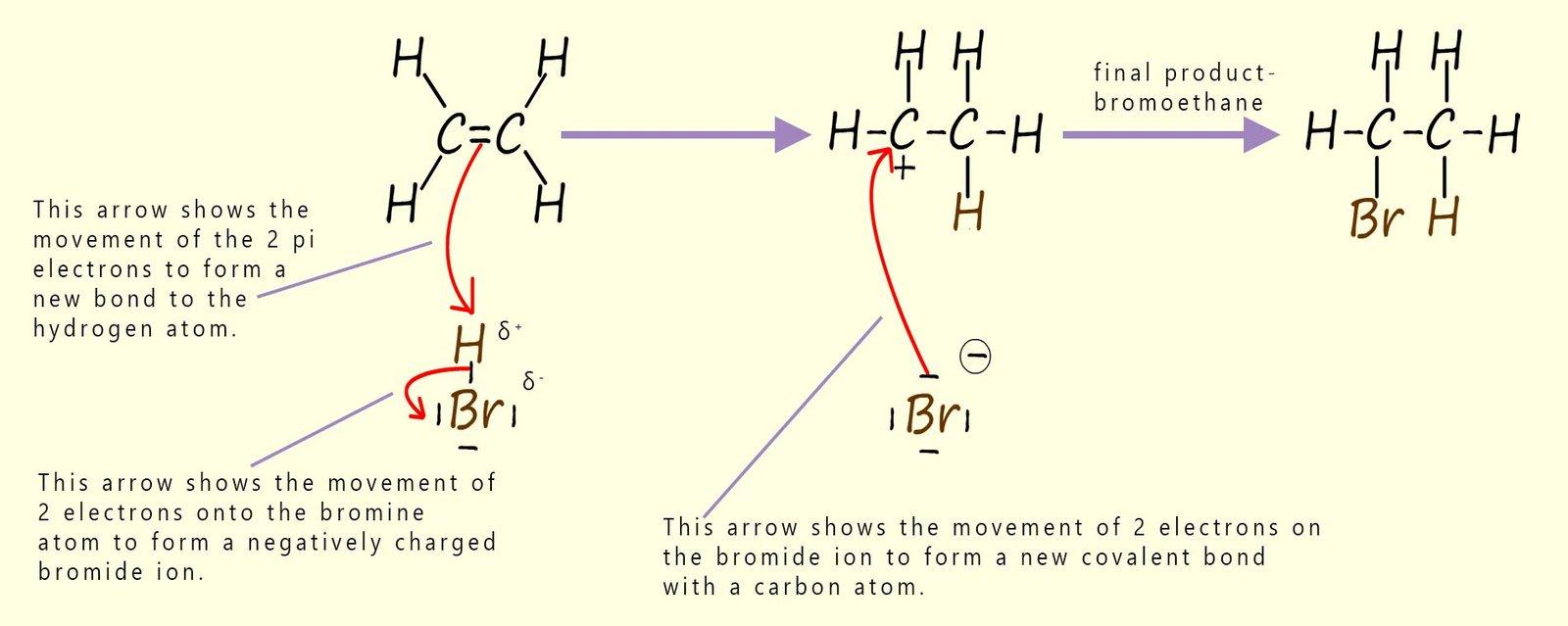
The steps below explain in detail what is happening at each step in this mechanism:
In the reaction mechanism shown above the partially positively charged hydrogen atom in the hydrogen bromide molecule acts as the electrophile, while the negatively charged bromide ion acts as a nucleophile when it attacks the intermediate carbocation.
Open the button below and answer the question below on curly arrows.
In electrophilic addition, curly arrows show movement of electron pairs. Answer this before you look back at the mechanism.
In the electrophilic addition of HBr to an alkene, where should the first curly arrow start?
✅ Exam habit: curly arrows start at an electron pair (a bond or a lone pair), not at a positive charge.
Note: The following work is NOT in the AQA specification but is examined by OCR/Edexcel.
Hydrogen chloride, hydrogen bromide and hydrogen iodide are all acidic gases and will readily dissolve in water to form acidic solutions.
These acids are all strong acids; the strongest acid is hydroiodic acid, followed by hydrobromic acid and then hydrochloric acid (HI(aq) > HBr(aq) > HCl(aq)). The mechanism we looked at above was for reactions taking place in the gas phase, so what would happen if the reaction was carried out in water (aqueous conditions) instead?
A very common mistake is to quote the same reactivity trend for all electrophilic addition reactions of hydrogen halides.
✔️ Always state the conditions before giving a reactivity trend.
Can you work out what will happen if we switch conditions for these electrophilic addition reactions from the gas phase to aqueous conditions?
Well the relative reactivity of these electrophilic addition reactions in aqueous solution is HI(aq) > HBr(aq) > HCl(aq). This mirrors the strengths of the three acids, where hydroiodic acid is a stronger acid than hydrobromic acid, which in turn is a stronger acid than hydrochloric acid. This is the opposite trend to that observed for the same reactions in the gas phase.
In aqueous solutions, the hydrogen ion (H+) from the acid acts as the electrophile in the reaction with alkenes and the halide ion acts as the nucleophile, attacking the intermediate carbocation to produce the halogenoalkane.
In aqueous solution the reactivity trend depends mainly on acid strength. This is because stronger acids such as HI(aq) dissociate more fully, producing a higher concentration of hydrogen ions (H+), which act as the electrophile that the pi electrons in the alkene attack. This step is the slow step in the reaction and determines how fast it proceeds. The halide ion acts as a nucleophile in a later step, but the overall reactivity is controlled by how readily the hydrogen ion (H+) is produced.
Click the button below to review your understanding of the differences in the reactivity of electrophilic addition reactions of the hydrogen halides in the gaseous and aqueous phases.
Click each card to flip it. Use this to test yourself on the key differences between gas phase and aqueous conditions.
In the gas phase the more polar H–X bond makes hydrogen more partially positive, so it is a stronger electrophile, but in aqueous solution the strongest acid produces hydrogen ions most readily.
Perhaps you were wondering how these reactions in aqueous conditions can actually take place, since alkenes are non-polar molecules and do not dissolve in aqueous acids. Instead; like oil and water, they form two immiscible layers. However although alkenes are insoluble in aqueous acids electrophilic addition can still occur at the interface between the alkene and the acidic solution. Insoluble does not mean unreactive!
Try the quick quiz below to review your understanding of the rates of reaction of the hydrogen halides in the gas phase and in aqueous conditions.
Predict first. Then click Check to see if you’re right (with a short reason).
Which hydrogen halide reacts most readily with ethene in an electrophilic addition reaction?
Now switch to aqueous conditions. Which reacts most readily with an alkene?
✅ Key takeaway: the trend can reverse when you change conditions.
Before moving on, tick the statements you feel 100% confident about. If you hesitate, that’s a sign to reread the section above.
✅ If you ticked most of these, you’re ready for exam questions. If not, scroll back — these are easy marks to secure.