


The bonds between atoms in molecules are a source of stored
potential energy.
To break a chemical bond you have to supply
energy, that is bond breaking is an
endothermic process.
However when the same chemical bonds are formed energy is released, usually as
heat, that is bond formation is an exothermic process.
The image opposite shows a small molecule of carbon tetrachloride (CCl4).
This small molecule contains 4 C-Cl covalent bonds, now
the bond enthalpy is the amount of energy
required to break chemical bonds and in the case of the C-Cl bond it is 336kJmol-1 and this same amount of energy will be released when 1 mole of C-Cl bonds are formed. The stronger the bond the more energy is required to break it and the more energy will be released when it forms.
The bond enthalpy for a diatomic molecule which is also often called the bond dissociation enthalpy is the enthalpy change for the following process where all the species are in the gaseous state:
The energy required to break a particular covalent bond
in an element such as hydrogen, oxygen or nitrogen are shown below:
The bond dissociation enthalpies listed above are for a particular bond
such as a H-H or a O=O covalent bond in a diatomic molecule. However many bonds such as C-C, C-H, C=O or O-H are found in many different types of
molecules and it is unlikely that an O-H bond in a water molecule would have the same
bond enthalpy as an O-H bond in say an alcohol molecule,
simply because the electron distribution within each O-H bond is bound to be slightly different,
and this will lead to different bond strengths.
So what value do we use for an O-H bond then?
Well as a work-around we gather data for O-H bond enthalpies from a large number of
molecules containing an O-H group and then take an
average or mean bond enthalpy across all these molecules.
However this means that if we use mean bond enthalpies when calculating
enthalpy changes for a reaction the result may differ slightly from any experimentally
measured value. The differences are usually minor and using mean bond enthalpies gives a good
estimate of the enthalpy changes taking place.
The mean bond enthalpy of a covalent bond is defined as:
The mean bond enthalpy is the average of many values of the bond dissociation enthalpy for a given bond found in a range of different compounds.

Consider the following process which shows the use of mean bond enthalpies in the breaking up of a molecule of methane in the gas phase to form individual atoms of carbon and hydrogen. To carry out this endothermic process requires an input of 1652kJmol-1 of energy.
Since this process represents the breaking of 4 C-H covalent bonds we can calculate the mean bond enthalpy of a C-H bond in methane as 413kJmol-1 (1652/4). It is unlikely however that all 4 C-H bonds in methane will require exactly the same amount of energy to break. For example once the first C-H bond has broken the remaining C-H bonds in CH3 and CH2 fragments will not have exactly the same bond dissociation enthalpy. Even so, using mean bond enthalpies will give a good approximation of the enthalpy changes taking place.
If you can follow this example, you can answer most A-level questions on mean bond enthalpies.
| Bond | Mean bond enthalpy/kJmol-1 |
|---|---|
| C-H | +412 |
| H-H | +436 |
| O=O | +497 |
| O-H | +463 |
| C-Cl | +336 |
| C=O | +743 |
| C=C | +612 |
The table opposite gives some common mean bond enthalpy values. These are mean values taken from a wide range of compounds. We can use this data to calculate the enthalpy change for reactions where there is no change of state. This usually means reactions in the gaseous state. As a simple example consider the combustion of methane gas to form carbon dioxide and water vapour:
To calculate the enthalpy change for this reaction we follow a series of simple steps:
If possible it is often very helpful to draw a quick sketch to show the molecular structure of the reactant and product molecules. This helps you see clearly all the bonds which are broken and formed during the reaction. The image below shows this for the combustion of methane:
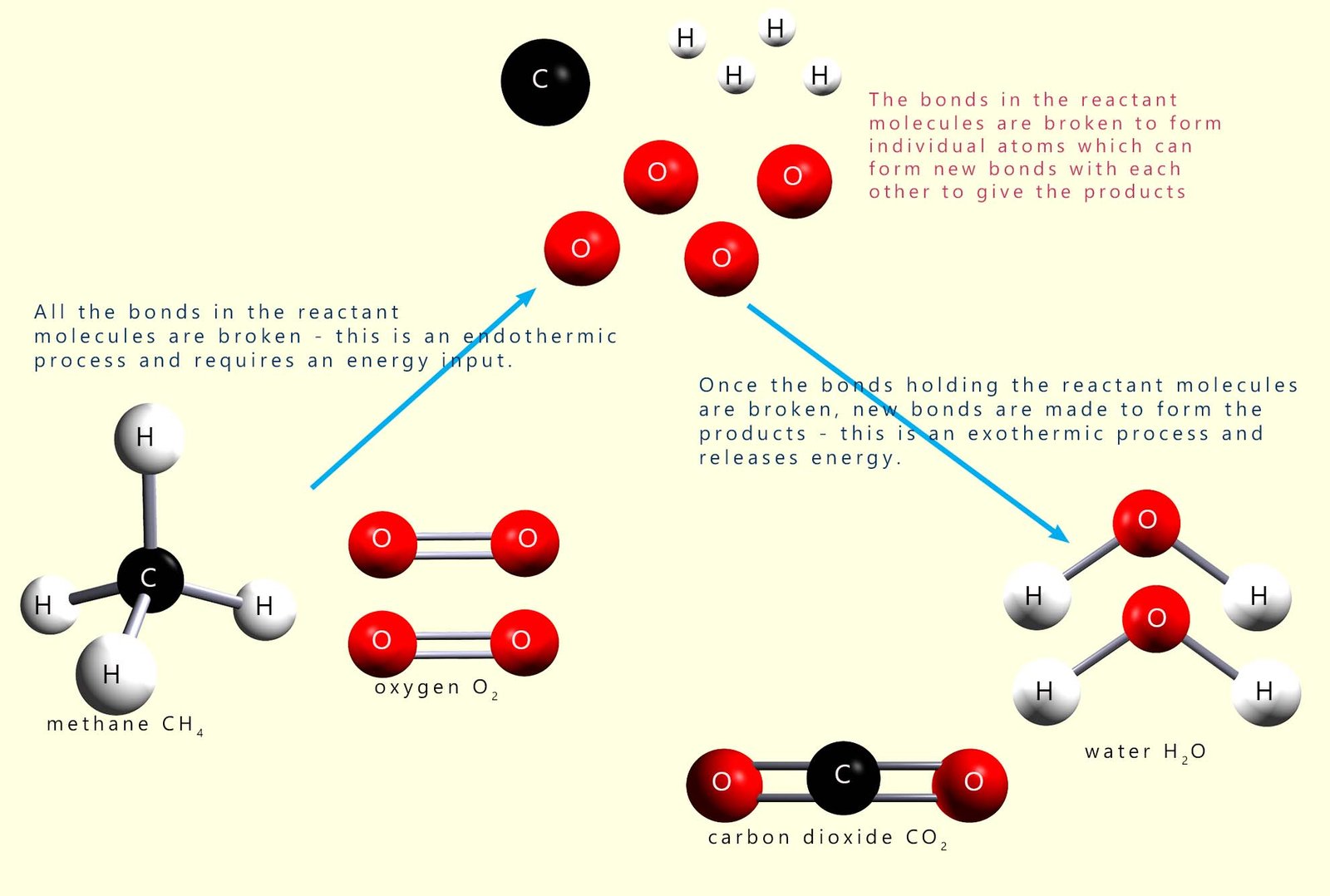
To calculate the enthalpy change for the reaction it is simply a case of adding up the individual mean bond enthalpies for all the bonds in the reactants which are broken and also for all the bonds in the products which are formed and then putting these values into the formula given, as shown in the table below:
| Bonds broken | Mean bond enthalpy/kJmol-1 | Bonds formed | Mean bond enthalpy/kJmol-1 | |
|---|---|---|---|---|
| C-H x 4 | 412 x 4 = 1648 | C=O x 2 | 743 x 2 = 1486 | |
| O=O x 2 | 497 x 2 = 994 | O-H x 4 | 463 x 4 = 1852 | |
|
energy supplied to break all bonds in the reactants: 1648 + 994 = 2642 |
energy released by bond formation in products: 1486 + 1852 = 3338 |
|||
|
ΔH = Σ(bond enthalpies of bonds broken) - Σ(bond enthalpies of bonds formed)
= 2642 - 3338 = -696kJmol-1 |
||||
Since in this example more energy is released by bond formation than is taken in by bond breaking, the reaction is exothermic with a negative enthalpy change (ΔH is negative).