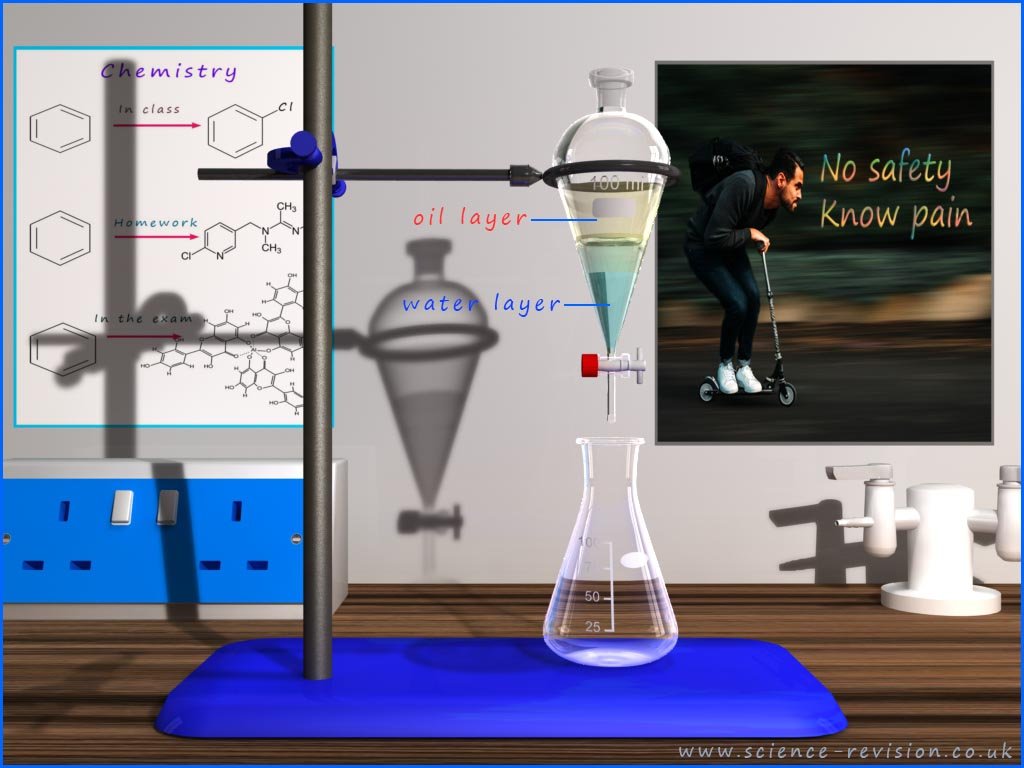
Distillation is used to separate a mixture of
miscible liquids; that is liquids that will mix
freely with each other. Some liquids like oil and water do not mix;
these are called immiscible
liquids. Oil is less dense than water so it floats on top. To separate these two
immiscible liquids you use a
separating funnel.
This is simply a "flask" with a tap at the bottom. In the example shown a thin
layer of oil is sitting on top of a layer of water. If you simply open the tap and the lower liquid
water layer will drain out. When all the water has drained out you simply close the tap and the oil
will be left in the separating funnel and the water will be in the flask, this is outlined below: