

Higher and foundation tiers
Chromatography is another method used to separate mixtures and also identify the substances present in
the mixture. The method outlined
below is called ascending paper chromatography.
Chromatography uses a mobile phase and a stationary
phase to separate out a mixture of substances. In the method shown below
chromatography is being used to separate out a
mixture of different coloured inks. Here the mobile phase is a liquid
solvent;
water or ethanol are commonly used as solvents in many chromatography experiments. The solvent soaks into the chromatography paper and dissolves the
mixture of substances in the coloured dots. Since the solvent
soaks into the chromatography paper and starts to move up the chromatography paper it is called the mobile phase. The
chromatography paper is called the stationary phase, it obviously does not move.
The image below shows a typical paper chromatography set-up. Here a pencil line; not a pen as the ink from the pen would run and spoil the chromatogram is drawn approximately 2-3cm from the bottom of the chromatography paper. This line is often referred to as the baseline. Capillary tubes are then used to spot the ink samples onto the chromatography paper which is placed in the chromatography tank. The paper should dip into the solvent but it is important that the solvent is below the pencil line or baseline. A lid is placed on the tank to keep the solvent vapours in. The solvent is allowed to rise approximately 75% of the way up the chromatography paper; the paper is then removed and allowed to dry.

In the example below three dots of coloured ink; red, yellow and green are
placed or spotted on the pencil line or baseline. As the solvent rises up the
chromatography paper it will reach each of the three coloured dots in turn; however it is highly likely that each of the substances present in the
coloured dots will dissolve by a slightly different amount, this is one of the key points in how chromatography works and how it is able to separate out the different coloured inks present in each of the coloured dots. If any of the substances in the dots are
insoluble they will simply stay on the pencil line or baseline. However the more soluble
a substance is the further up the
chromatography paper the solvent
will carry it.
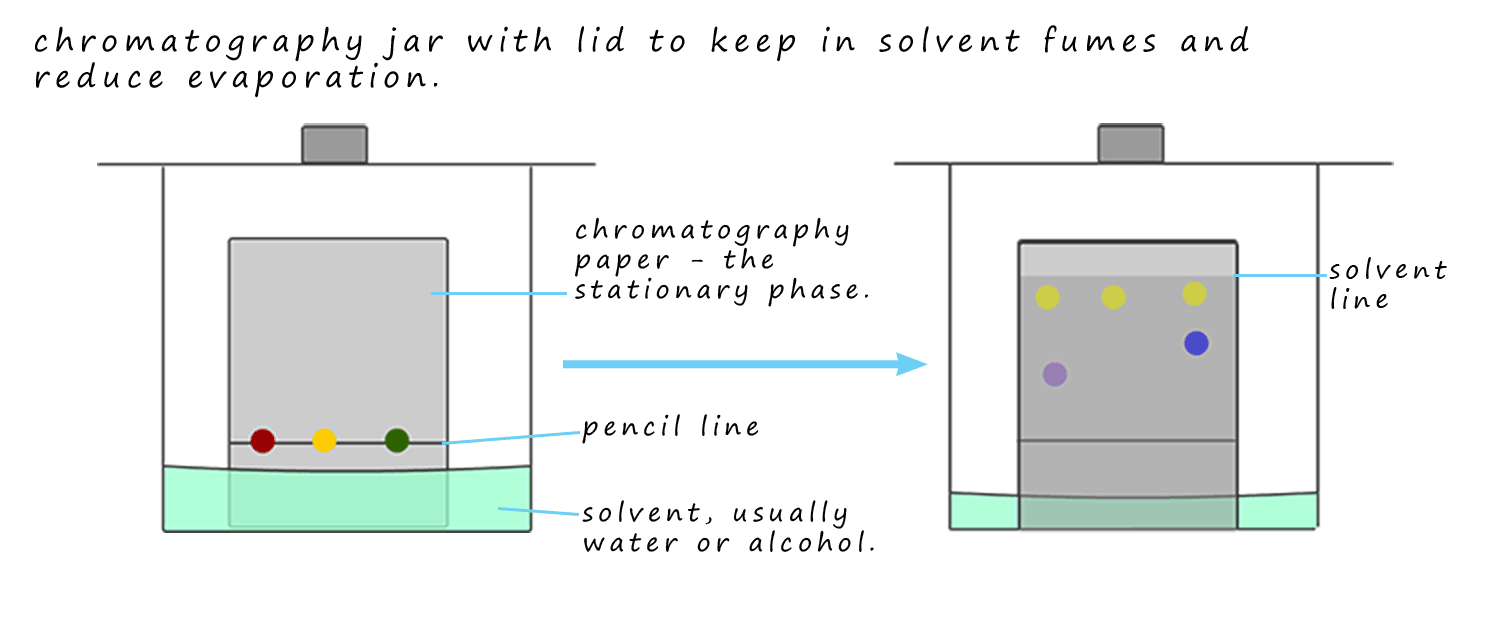

Another way of stating this is: the more time the substance spends in the mobile phase; that is dissolved in the solvent and the less time it spends in the stationary phase the further up the chromatography paper it is carried. When the solvent has risen close to the top of the chromatography paper it should be taken out of the tank and left to dry.
Once the chromatogram has dried out it is possible to measure how far each of the substance has moved through the stationary phase (travelled up the paper!). All measurements are taken from the pencil line or baseline. This is shown in the diagram below.
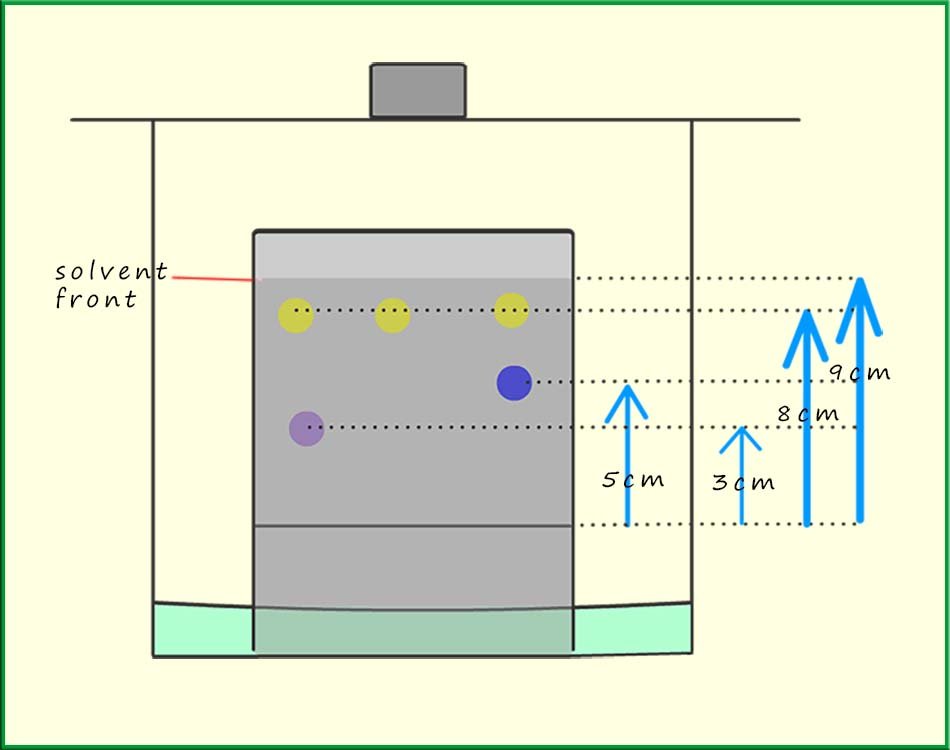
The retention factor or Rf value can be calculated for each substance. The Rf value is simply the ratio of how far the substance or solute moves divided by how far the solvent (mobile phase moved). It is calculated using the formula below:


The red ink in the chromatogram opposite produced two dots on the chromatogram; one dot was
magenta and one yellow.
The fact that there were two dots tells us that the ink colouring
is not a pure substance but that
it is a mixture of two other chemicals. The Rf value for the magenta dot is calculated as follows:
Rf= 3cm/9cm =0.33
The green ink also produced two dots on the chromatogram;
so like the red ink it also
is not a pure substance but it consists of two other coloured chemicals; one blue
and one yellow. The
Rf value for the blue dot/colouring would be:
Rf = 5cm/9cm=0.55
It should be noted that if the solvent, temperature or type of
chromatography paper used in a
chromatography experiment is changed then the Rf values will also change.
The Rf values are ONLY true
if the same conditions are used for repeated experiments. However if two substances have the same
Rf values under the same conditions it is highly likely they are the same substances. This is how
chromatography can be used to identify unknown substances.
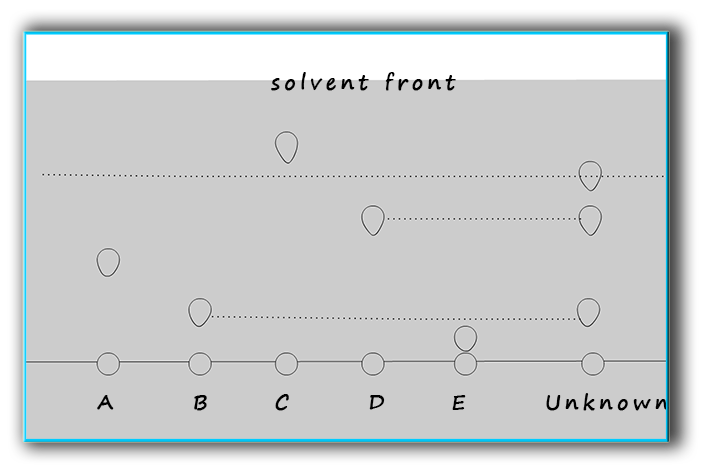
Chromatography can also be used to identify substances. You can compare the Rf values for known substances with your unknown e.g. The chromatogram below shows 5 known substances A, B, C, D and E. Each of these known substances are pure as only one "dot" or "smudge" was produced on the chromatography paper. The unknown substance is composed of 3 separate substances since three dots were produced on the chromatography paper. Two of the three substances in the unknown match up with substances B and D and a third substance in the unknown mixture is still unidentified as it matched up with none of the other known substances.
Match the terms below with their correct defintion by simply clicking on the term and then its correct defintion. Correct responses will turn the defintions green!
