

Higher and foundation tiers
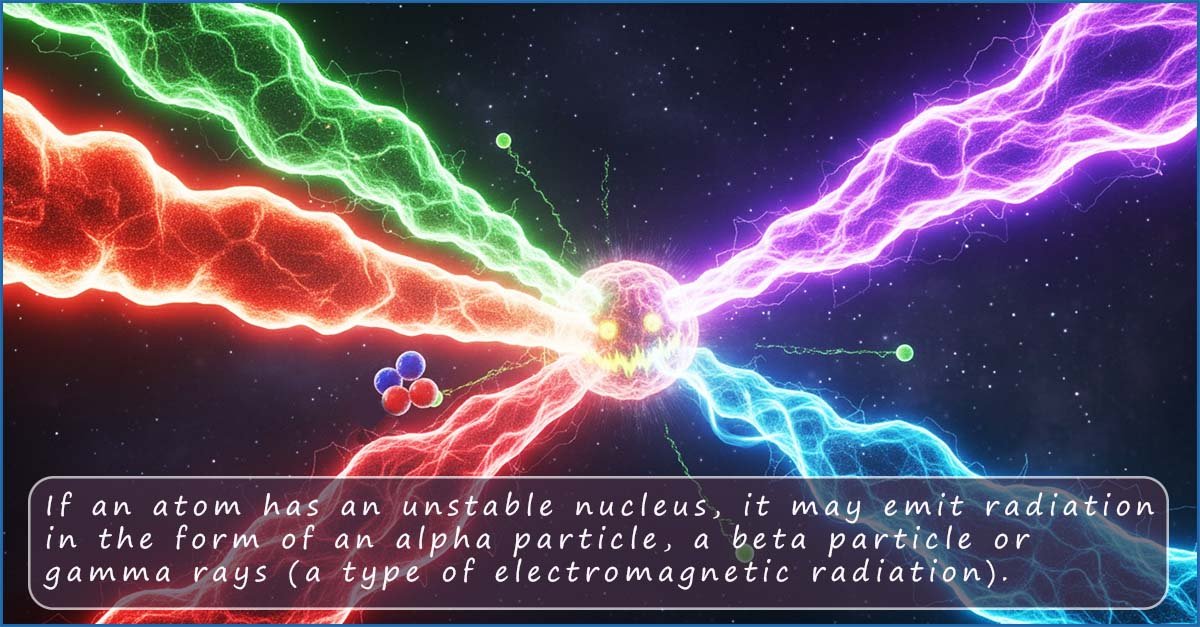
You are no doubt aware that the noble gases are chemically unreactive elements; this lack of chemical reactivity is due to the fact that they have full outer electron shells. However for the rest of this page we will focus on another property of atoms that has nothing to do with their chemical reactivity; that is whether an atom of a particular element is radioactive or not. Whereas chemical reactivity depends entirely on the number of electrons present in the outer or valence electron shells radioactivity is a phenomenon that depends on whether the nucleus of an atom is stable or not.
An atom can be chemically inert or unreactive but be highly radioactive. Radon is such an element, being a noble gas it is chemically unreactive but it is highly radioactive. In fact radon gas is the single largest source of background radiation that most people are exposed to. Unusually for an element all the isotopes of radon are radioactive it contains no stable isotopes; every isotope of radon gas is unstable and it undergoes radioactive decay.
Many of the isotopes of radon have half-lives that range from a few hours to a few seconds; however the most stable and most worrying from a public health point of view is radon-222 (222Rn); this particular isotope has a half-life of about 3.82 days. Radon-222 emits alpha particles (α), which is the most damaging form of radiation to human tissue when inhaled or ingested. When inhaled alpha particles (α) can damage the DNA in the lung cells and long-term exposure significantly increases the risk of lung cancer. Radon is in fact the second leading cause of lung cancer globally, after cigarette smoking and is one of the major causes of lung cancer among non-smokers.
Radon gas is produced by the radioactive decay of certain heavy metals such as uranium and thorium which are found in many rock types and also in the soil. An isotope of radon such as radon-222 with a half-life of 3.82 days will "hang-around" long enough in the soil to rise from the soil where it forms and enter into homes and buildings through gaps or cracks in the walls and foundations; the air pressure inside homes and buildings is usually slightly lower than in soils and so this will help "suck" the radon gas into the building. Outdoors, radon is usually diluted to very low levels; but problems arise mainly indoors where concentrations can build up particularly in basements and cellars where the heavy dense radon gas can collect and its concentration can build up to dangerous levels.
Oddly the primary health risk from radon gas comes not from the inhaled radon gas itself; which is often simply exhaled, but from the tiny particles created when radon undergoes further radioactive decay inside the home. When radon undergoes radioactive decay it produces isotopes of heavy metals such as polonium (Po), lead (Pb), and bismuth (Bi). These solid metal particles can attach themselves to dust, smoke, and aerosols in the air and when these contaminated particles are inhaled, they become trapped in the airways and lungs. Once lodged in the lung tissue, they continue to undergo radioactive decay, emitting highly damaging alpha particles (α). It is these alpha particles that can damage the DNA in the lung cells and long-term exposure significantly increases the risk of developing lung cancer.
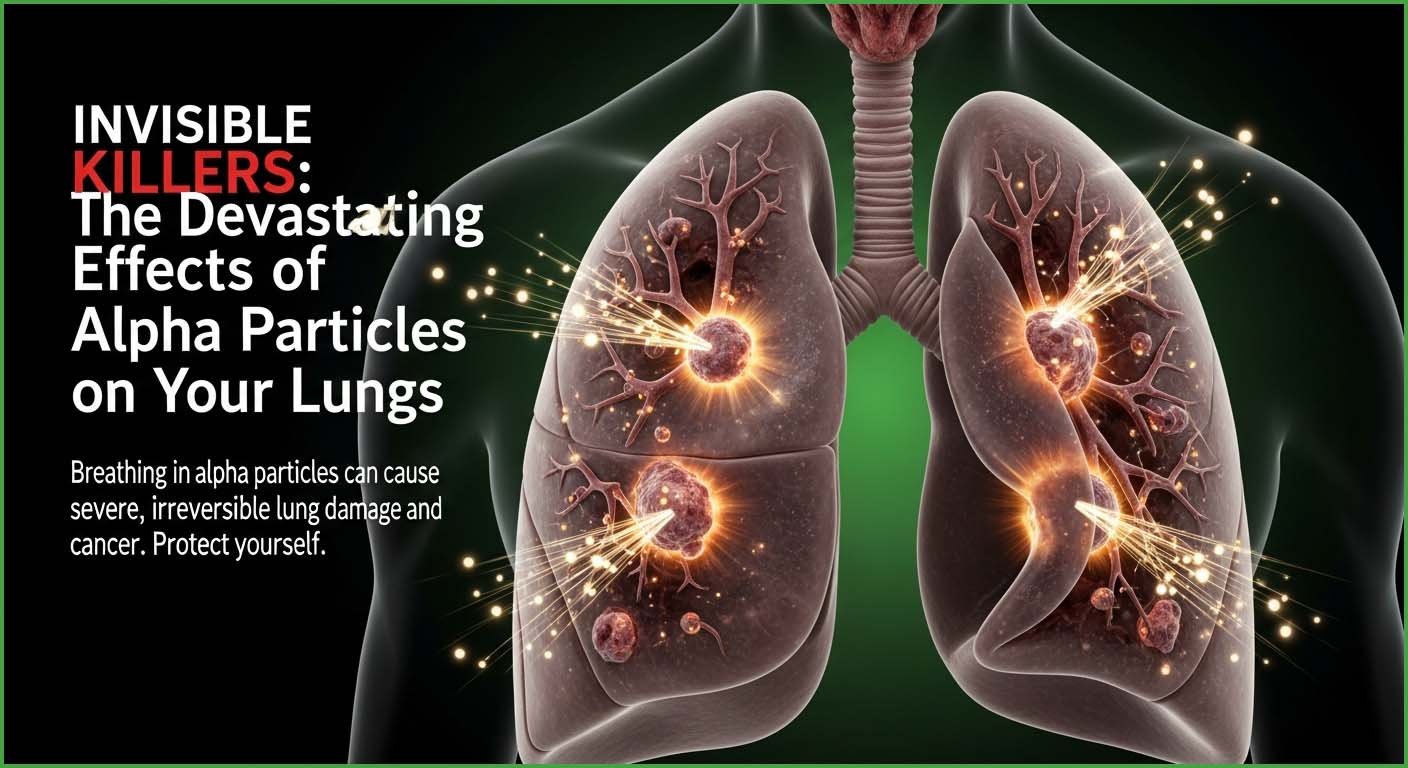
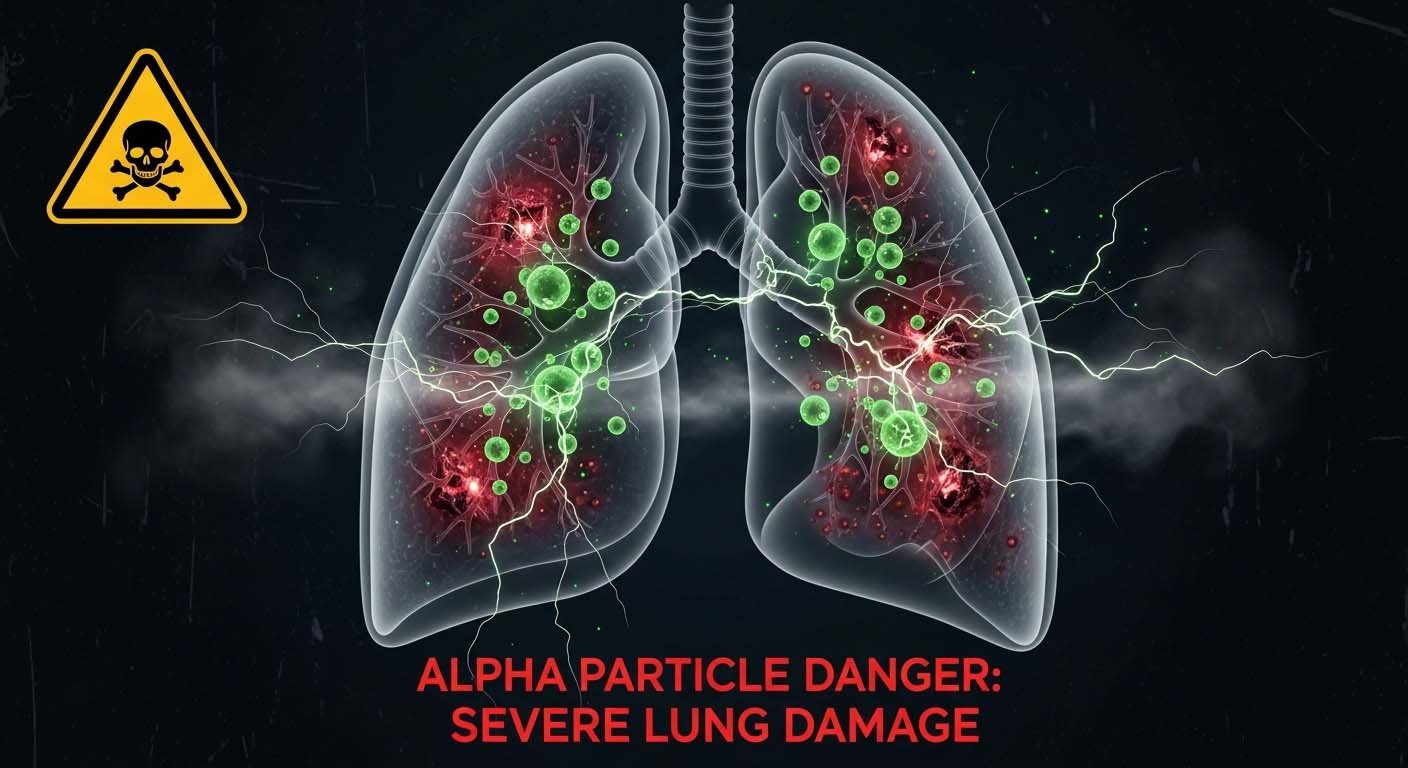
There is no known safe level for exposure to radon gas and it may take many years before the symptoms of cancers begin to appear; so perhaps one of the most important steps to take is to regularly monitor and test the levels of radon gas in your home; recall that radon is a naturally occurring, colourless, odourless, and tasteless radioactive gas so it is essential that you monitor levels in your home especially if you live in an area where levels of the gas are known to be high.
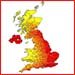
Radon levels vary across the UK due to differences in the local geology. Some areas for example parts of Cornwall, Devon, Derbyshire (Peak District), Wales, Northern Scotland and Northern Ireland have a higher proportion of homes above the UK average level. Every home is different, though, so the only way to know your level is to check and test.
Use the flash cards below to quickly review your understanding of some of the main points covered above. Tap a card to flip it.


Click the cards below to build a paragraph to explain how radon forms and the dangers it poses.