

Chemistry only
The main part of the periodic table is shown in the image below. The centre part (coloured red) contains the transition metals. If you were to ask most people to describe a metal the chances are they would be describing one of the transition metals; probably silver, gold or iron. The transition metals have the properties most people associate with a metal.
When compared to the alkali metals in group 1 of the periodic table the transition metals are:
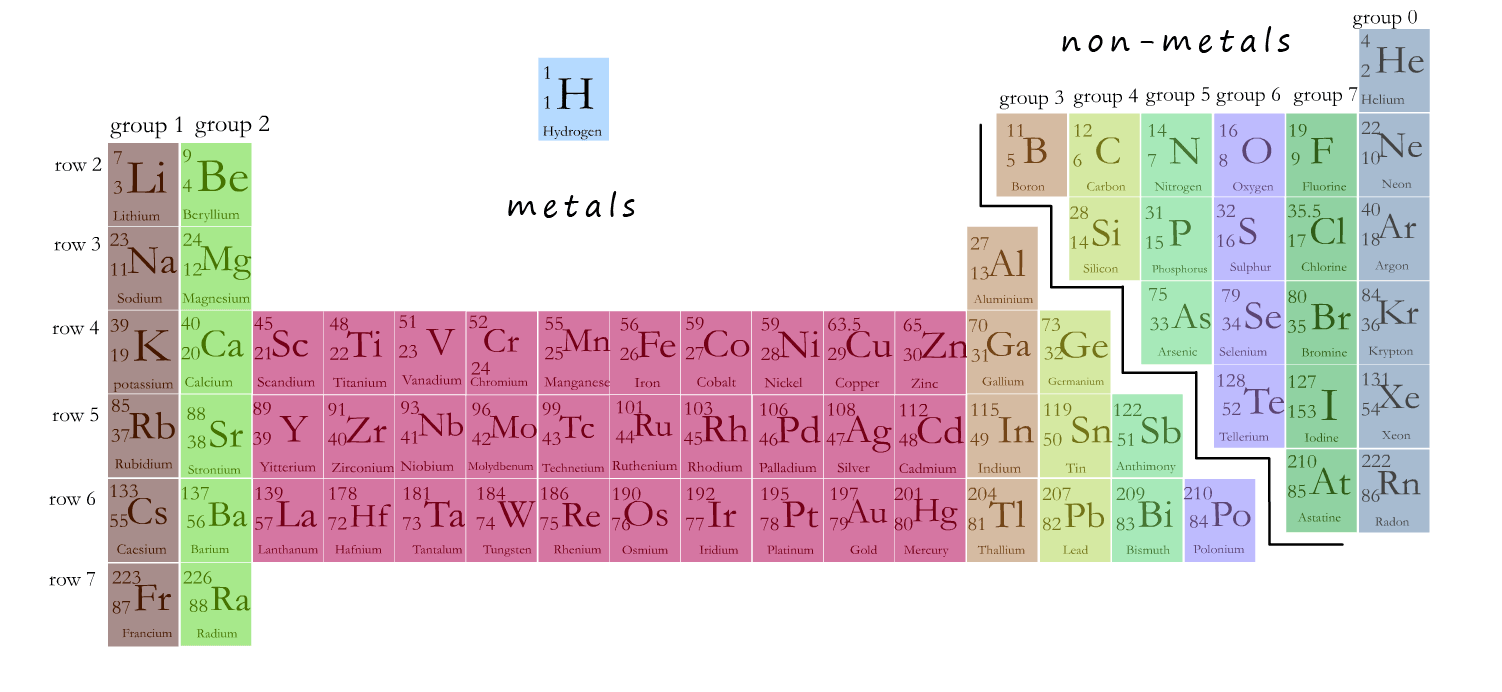
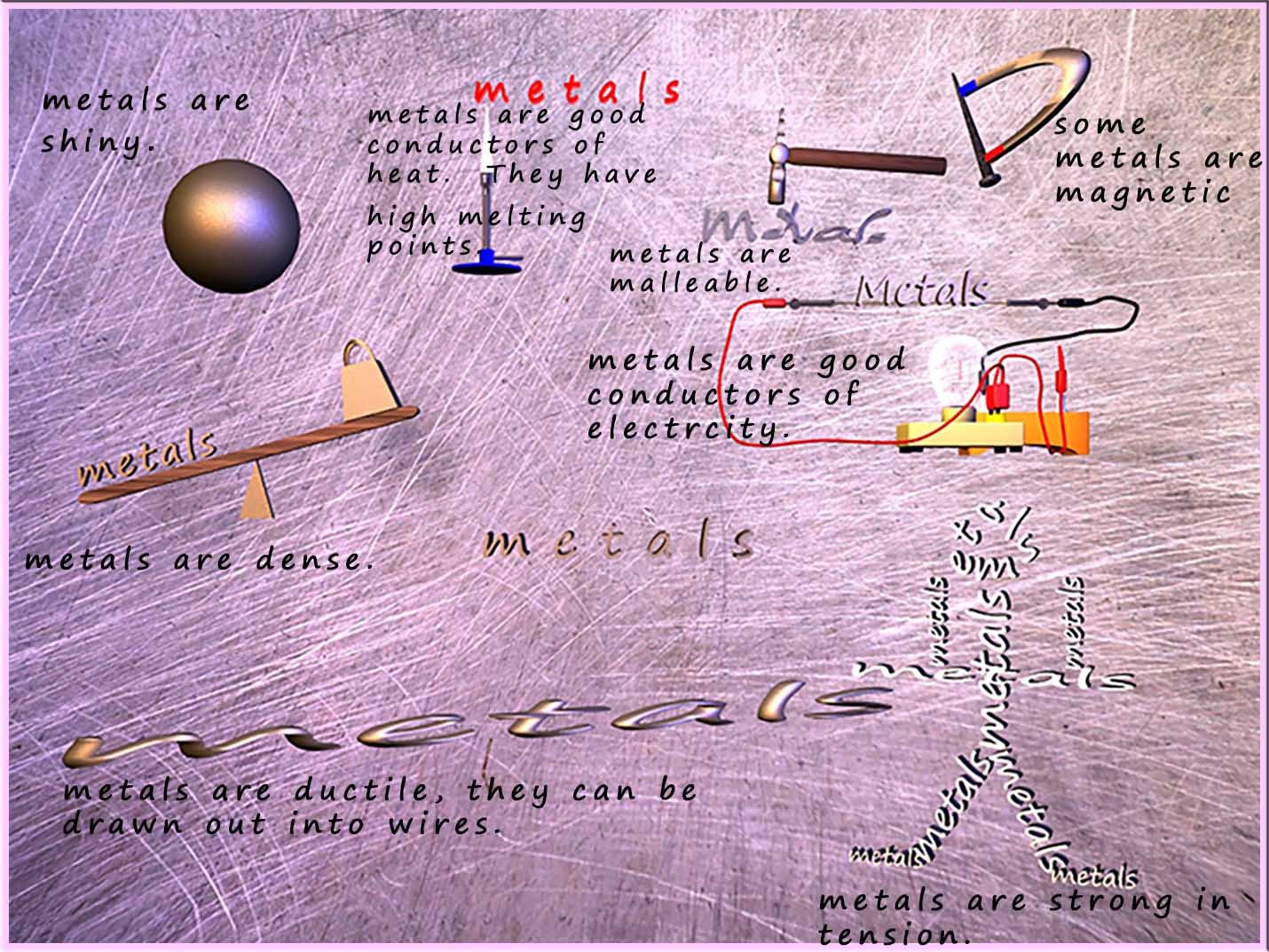
The common properties which most people associate with metals are summarised in the image below. (click here to view more on metals)


I am sure you are aware that metals when they react lose electrons, so for example a group 1 alkali metal will lose 1 electron and form an ion with a 1+ charge, similarly group 2 and group 3 metals will lose 2 and 3 electrons respectively to forms ions with 2+ and 3+ charges.
The transition metals are different in that they can have variable valencies; that is they can lose different numbers of electrons depending on what they react with and on the reaction conditions e.g. Iron atoms can lose 2 or 3 electrons to form ions with a 2+ or 3+ charge. Copper can lose 1 or 2 electrons to form ions with a 1+ or 2+ charge, some metals such as manganese can form ions with charges that range from 2+ to 7+. The amazing thing is that the ions with different charges also different colours.
Transition metal compounds tend to be brightly coloured compounds whereas compounds containing metals from group 1 and group 2 are mostly colourless (white). The image below shows the wide range of colours for the transition metals manganese (present in permanganate), cobalt, chromium (present in dichromate and chromate), nickel and copper. Many of these coloured compounds containing transition metals are used to colour ceramics such as plates, pots and cups. You will also find many coloured transition metal compounds used in paints, glazes and dyes.

Many transition metals have been known for thousands of years and most have the properties we
associate with metals. Transition metals such as chromium, titanium and tungsten are very hard
metals used in many tools and machines. Transition metals have high melting points, with the
exception of mercury which is a liquid at room temperature. Tungsten is a transition metal with the second highest melting point of
any element in the periodic table, a jaw dropping 34220C. Transition metals are also good
electrical conductors with copper being used in wires and gold and silver being used in circuit boards in high end
electronic devices.
Transition metals are also malleable; that is they can be hammered, rolled, pressed and
beaten into different shapes without breaking. Most transition metals are also ductile, that is they can be drawn
out and stretched into wires. Copper, gold and silver are ductile metals commonly used in wires for
such things as HDMI connectors for TV and other electronic devices.
Iron another transition metal is the main ingredient in steel. Steel like most transition metals
has high tensile strength; this means it can support a heavy load before breaking.
Many transition metals are used as catalysts in industrial processes to speed up and increase the efficiency of the reactions taking place, iron, nickel, platinum, rhodium and palladium are transition metals which are commonly used as catalysts. Many transition metals are essential in the development of new batteries needed for electrical cars and modern electronic devices while other metals such as titanium are strong light weight metals that are used in the aerospace industry; the images opposite provide more detail on the uses of some transition metals but a quick search on-line will quickly provide you with any additional information you may need.
Common examples of catalysts that you will likely meet in your studies include: the use of an iron catalyst in the Haber process for making ammonia. Platinum, palladium and rhodium are the main transition metals used as catalysts in catalytic converters in cars. These catalytic converters are very effective at reducing harmful emissions produced by unwanted reactions inside car engines. Nickel is also a transition metal that is widely used as a catalyst; for example it is used in the hydrogenation of vegetable oils; this "hardens" the vegetable oil and turns it into margarine.
Test your understanding of the main properties and uses of transition metals by completing the paragraphs below. Simply select the correct word from the drop-down menus to complete the paragraphs. To help you complete the sentences all the words in the drop-down menus are shown in the yellow boxes.
