

Chemistry only

You should know what addition polymerisation is and how addition polymers are formed before you read this page. Click here to watch a quick video to revise this topic; this will help you understand the examples shown below:
The simplest and perhaps best known addition polymer is poly(ethene) (also called polythene). There are many common everyday items made from poly(ethene), including:
Poly(ethene) (or polythene) is made by joining many small ethene molecules (the monomer) to form the polymer chain. This can be represented by the model and displayed formula below:
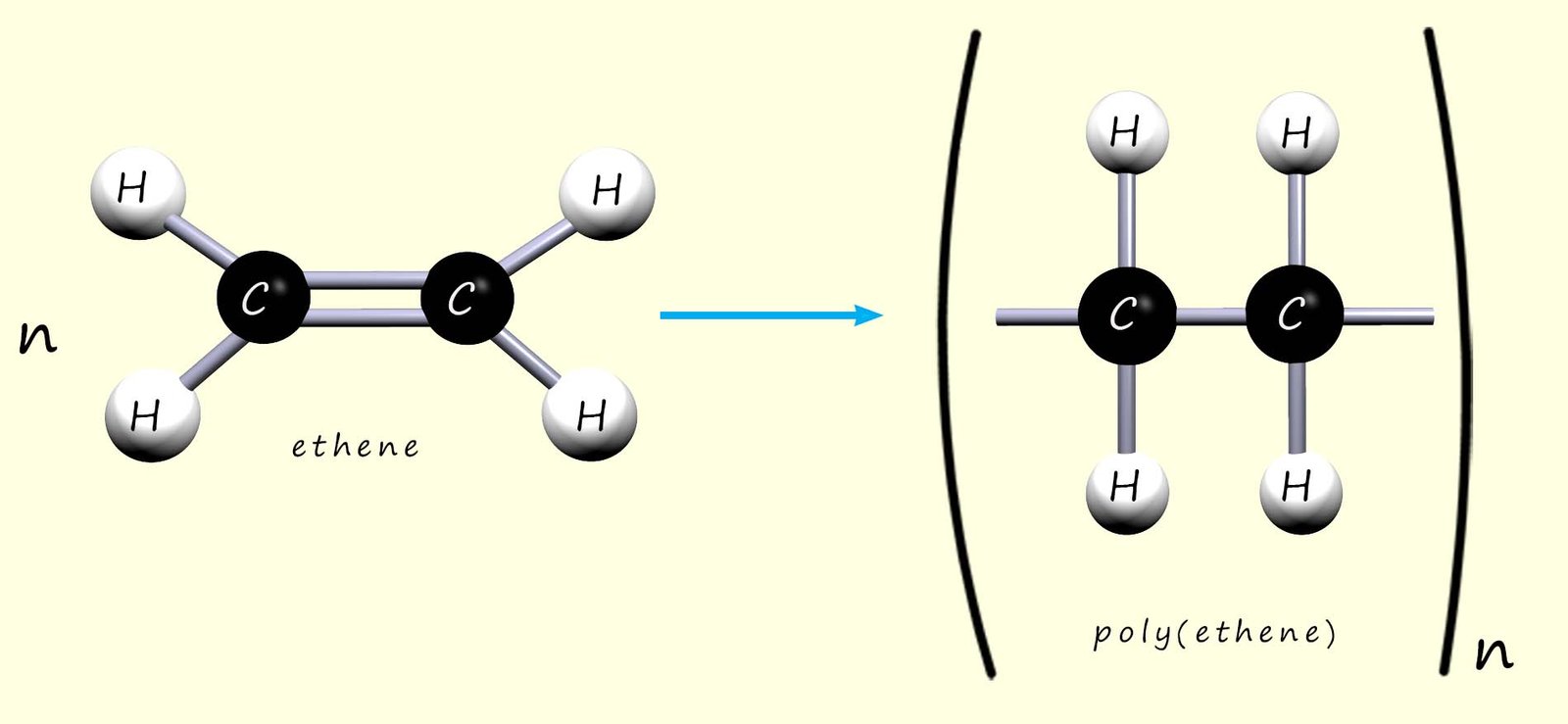
Or we can write an equation to show how ethene polymerises to form poly(ethene):
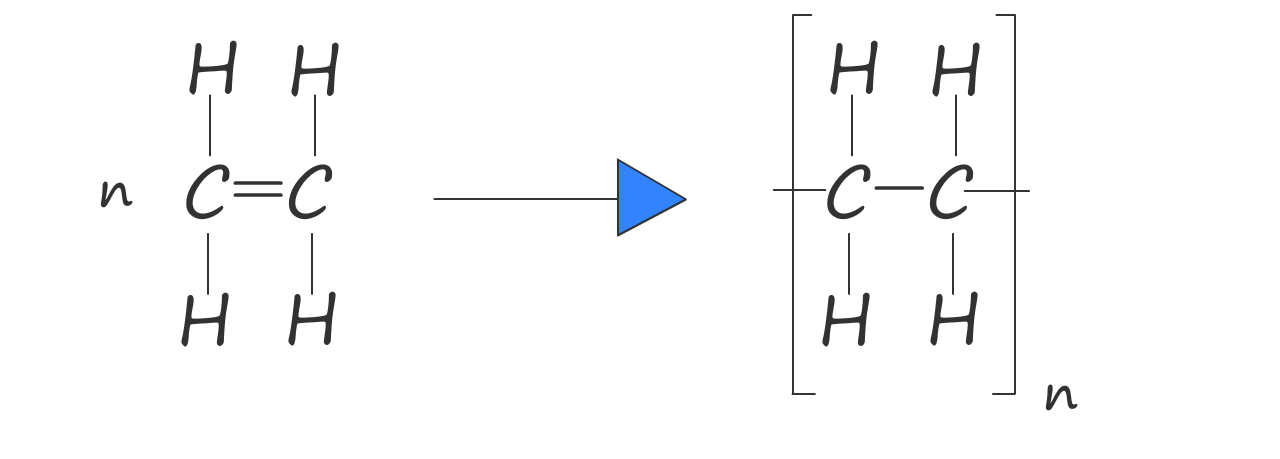
Here n represents the number of ethene molecules (or monomers) in the polymer chain.
All addition polymers are made in the same way as shown above for poly(ethene); different polymers are made by starting with different monomers, e.g.:

By replacing one of the hydrogen atoms in an ethene molecule with a chlorine atom, a molecule called chloroethene is produced. Chloroethene is an unsaturated molecule and, like ethene, it can be polymerised. During polymerisation the carbon–carbon double bond (C=C) breaks and the reactive intermediates link to form the polymer. To name the polymer formed, add the word poly to the name of the monomer in brackets, e.g.:
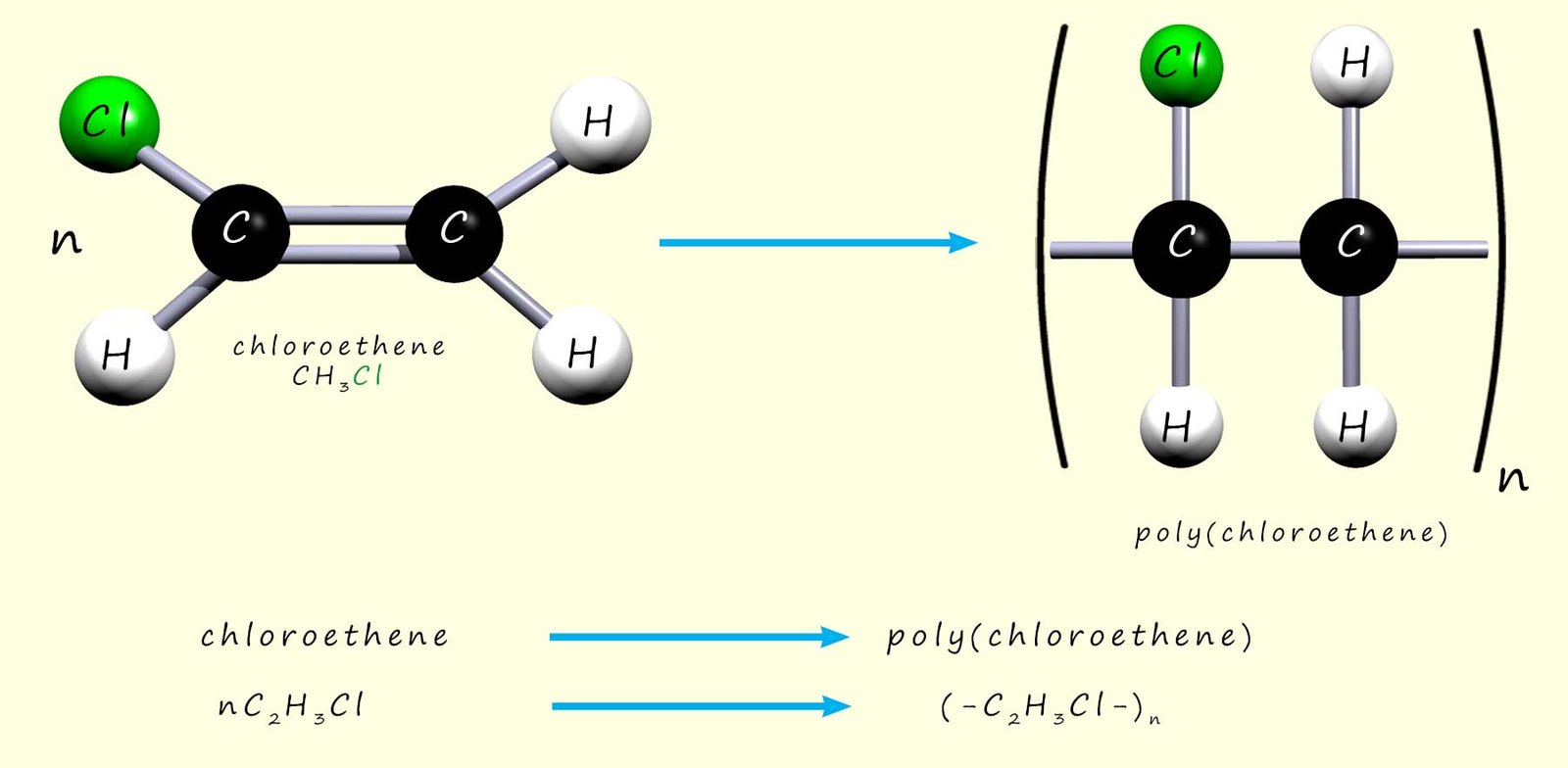
Or, as above, we can write an equation to show the polymerisation of chloroethene to form poly(chloroethene):
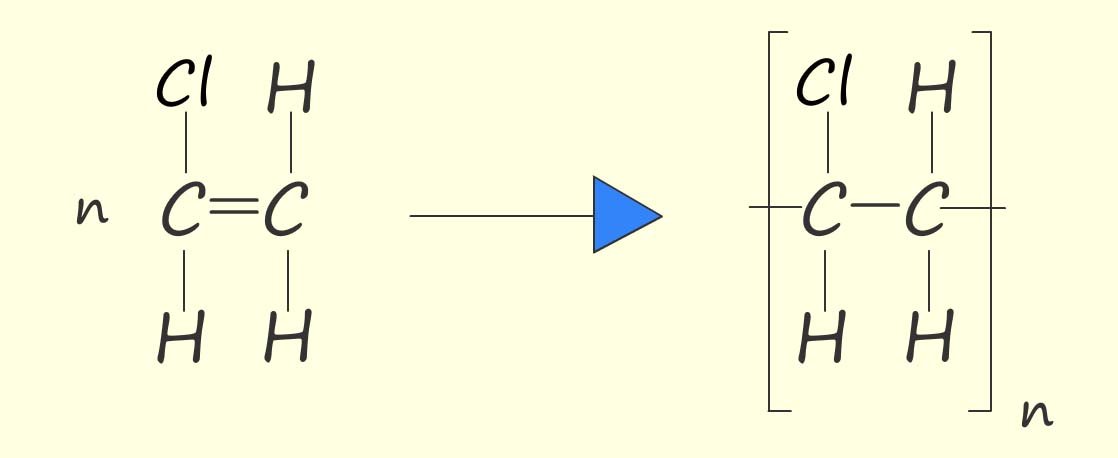
Chloroethene is the modern name of this molecule; traditionally it was also called vinyl chloride. So the polymer poly(chloroethene) was often called poly(vinyl chloride) or PVC. It is used to make items such as window frames (uPVC), guttering and drain pipes, cable insulation, flooring, and waterproof coats and boots.

In the example above we replaced one hydrogen atom on ethene with a chlorine atom to make chloroethene. If we replace all the hydrogen atoms in ethene with fluorine atoms, the new molecule is called tetrafluoroethene (“tetra” = four; “fluoro” = fluorine):
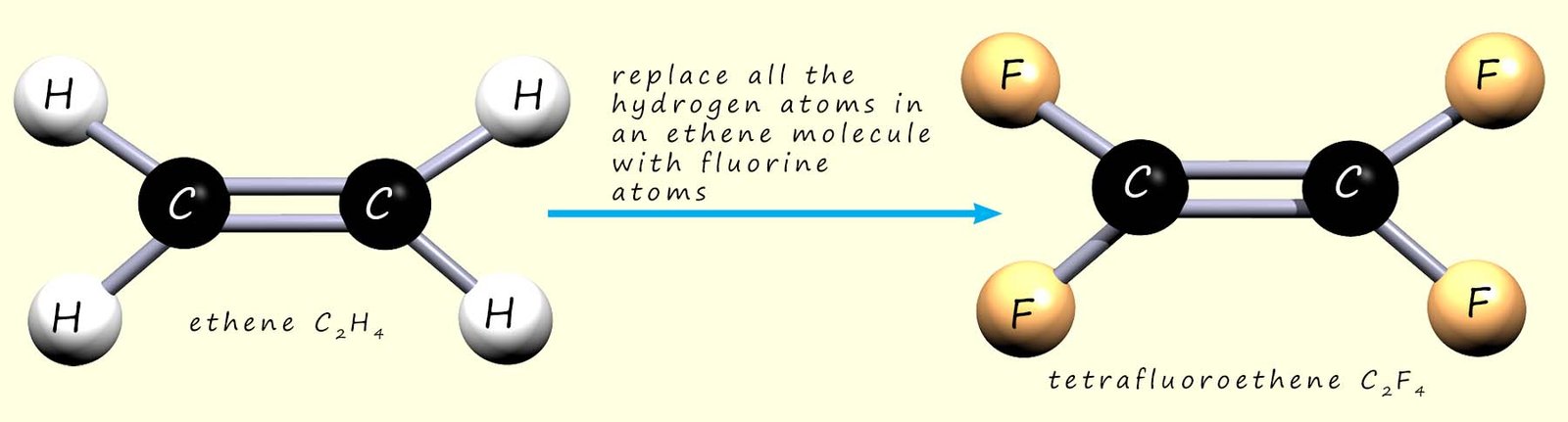
We can show the polymerisation of tetrafluoroethene to give poly(tetrafluoroethene) (PTFE, sold under the Teflon™ brand). As with addition polymerisation, the C=C bonds in the monomers break and the molecules link together:
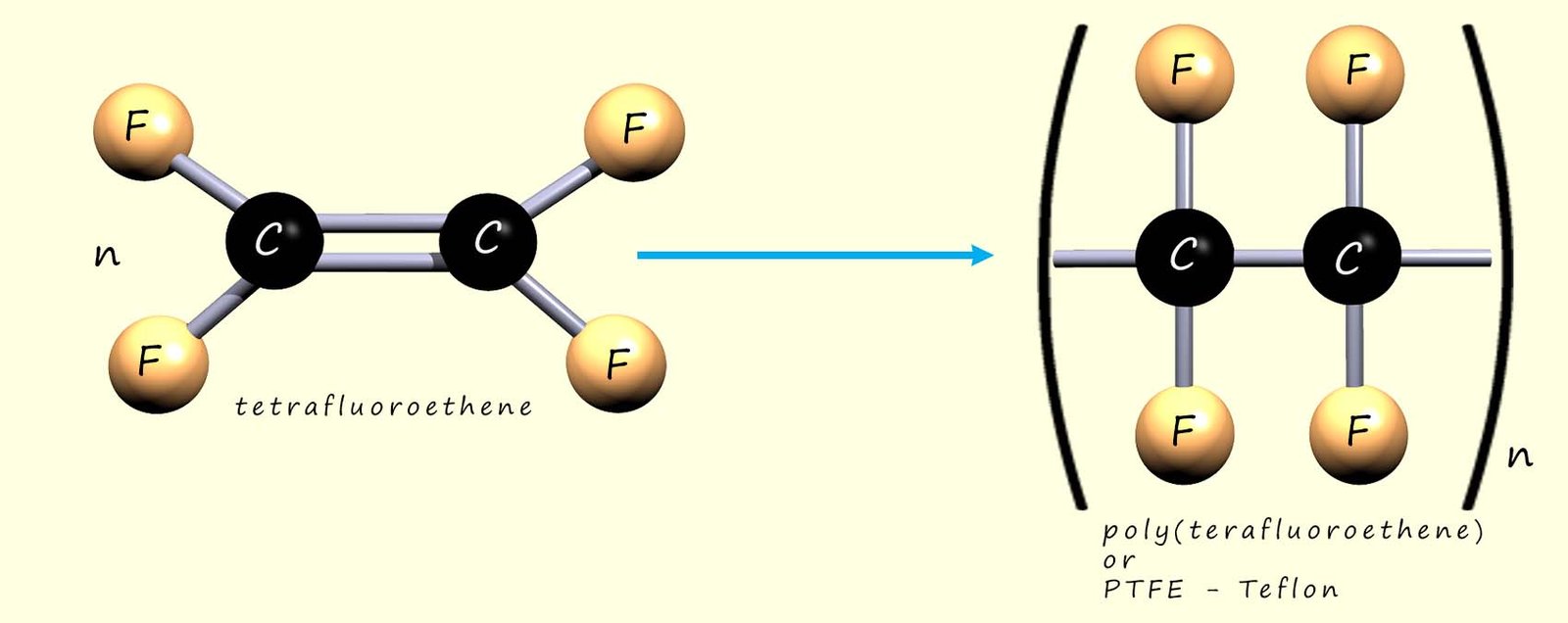
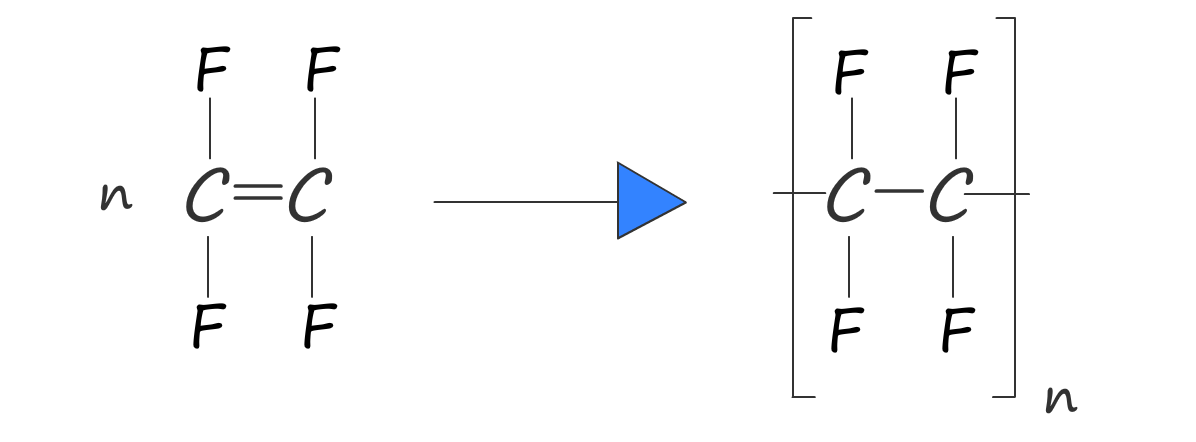
Or we can write an equation to represent this polymerisation reaction:
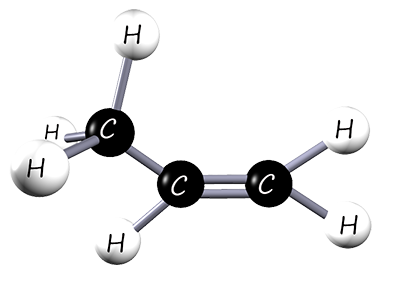
Poly(propene) (polypropylene) is widely used, e.g. in fibres for carpets, ropes and clothing; also car bumpers, milk crates and fishing nets. It is made from the monomer propene, an unsaturated alkene. The structure of propene is shown below:
| 3D model of propene | Displayed formula | Structural formula |
|---|---|---|
 |
 |
CH3CH=CH2 |
| C3H6 | C3H6 | C3H6 |
We can show the polymerisation of propene to form poly(propene) in exactly the same way as for ethene, tetrafluoroethene and chloroethene:
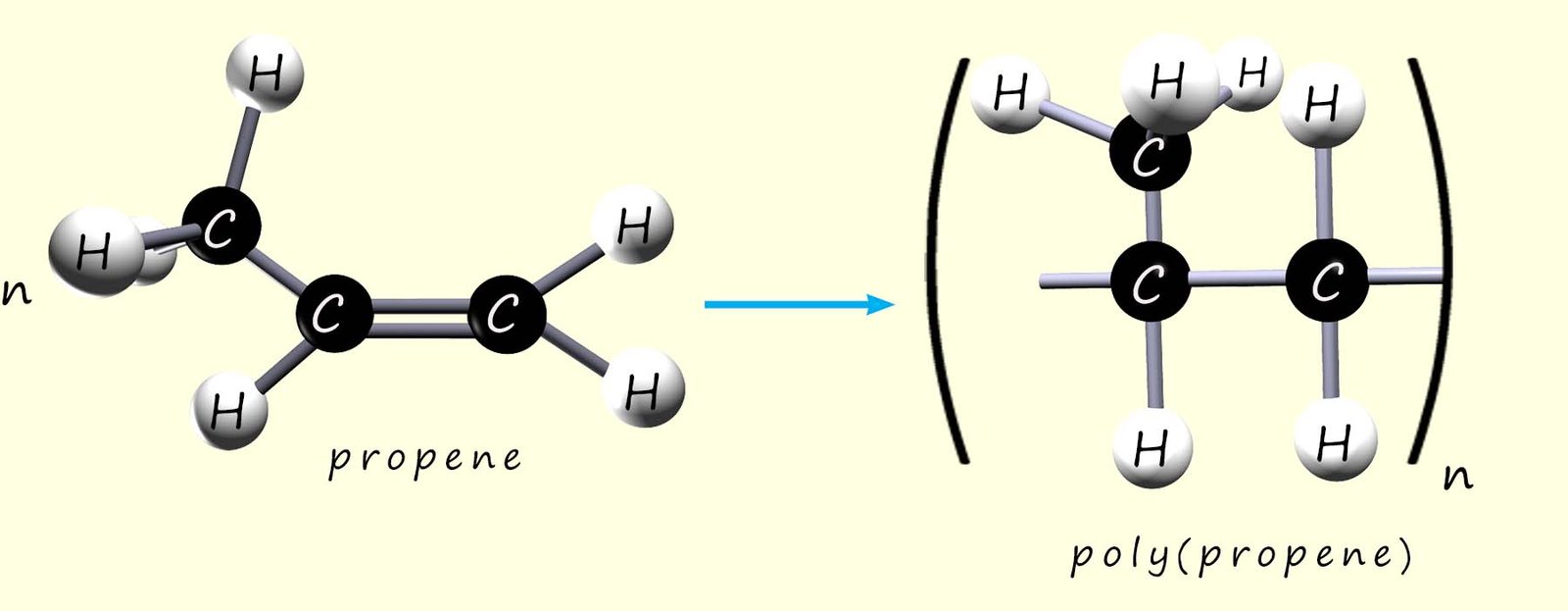
As before we can write an equation to represent this polymerisation reaction:

We can also represent the polymerisation using word and symbolic equations:
Review your understanding by completing the activity below. Select the monomer, add monomers to grow the chain, reveal the repeat unit, then name the polymer.
Choose a monomer, click "Add monomer" to grow the chain, then reveal the repeat unit and name the polymer.
poly(monomer) → poly(ethene).
Or in general we have:
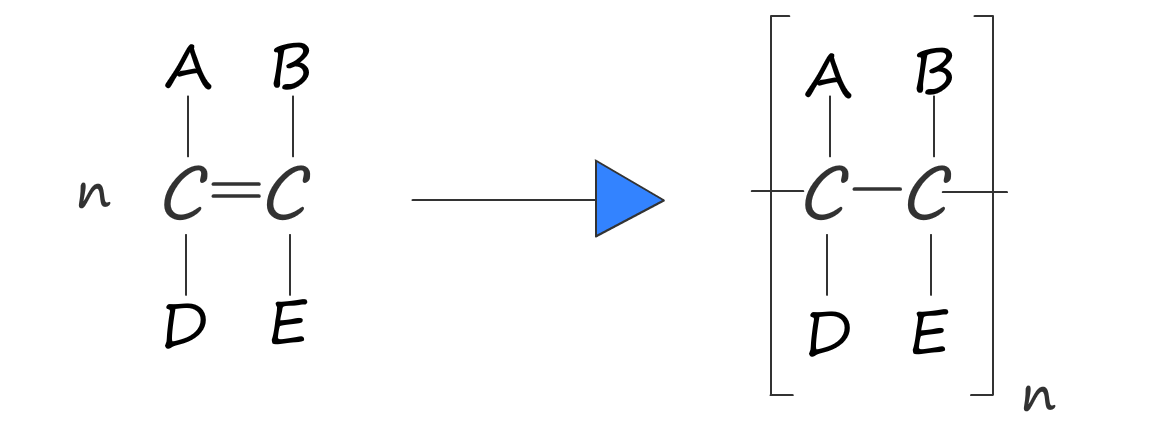
poly(monomer) — e.g. ethene → poly(ethene).n; break the double bond and add end bonds.