
Chemistry only- higher tier
From studying the page on polyesters we looked at how ester linkages could be formed in a condensation reaction; that is a reaction where two or more small molecules combine to form a larger molecule and release a small molecule such as water (H2O) or hydrogen chloride (HCl) gas. In the case of polyesters the two small molecules or monomers which reacted together to form the polymer polyester were a dicarboxylic acid and a diol.
Polyamides are also condensation polymers formed in a similar way to polyesters however this time the monomers used to make the polyamide polymer are a dicarboxylic acid and a diamine to form an amide bond. Common examples of polyamides include the polymers nylon and Kevlar, which are strong, durable materials used in clothing, ropes, and protective gear.
Amines are molecules containing the amino functional group (-NH2); so diamines are molecules containing two amino groups. The formation of a typical polyamide using a dicarboxylic acid and a diamine is outlined below:
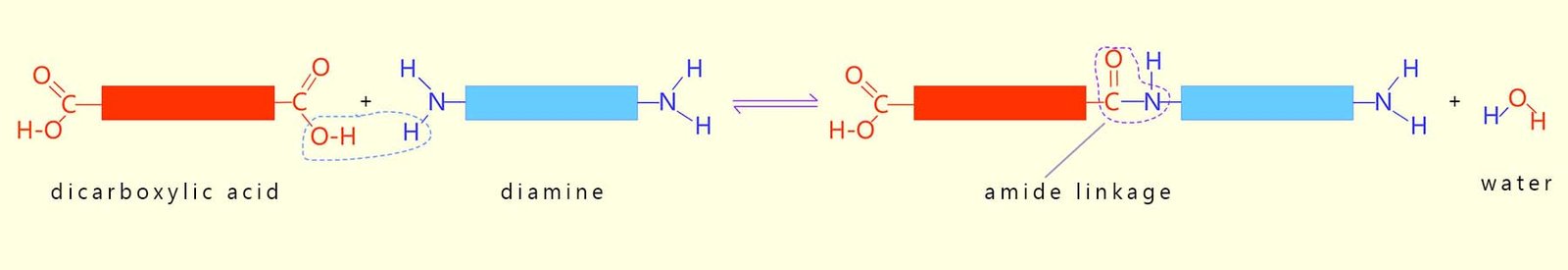
It is important to note that the product of the above reaction still has a reactive amino group (-NH2) and a reactive carboxyl group (-COOH) present on the ends of the new larger molecule formed, this means that the reactive amino and carboxyl functional groups present can react further and build up an even larger molecule. Or to put it another way the small dicarboxylic acid and diamine monomers can react together to build up a larger molecule containing lots of amide groups, that is a polyamide polymer can be made.
One of the first synthetic polyamides to be produced was nylon. Nylon was first made in the 1930s by Wallace Carothers while he was working for the American pharmaceutical company DuPont. One of the first uses for this new wonder material was to make ladies stockings. Ladies stockings had previously been made of silk; which unfortunately made them very expensive and not particularly hard wearing or long lasting.
When the first nylon stockings went on sale in America they were so popular that millions of pairs sold in only a few hours and shops quickly ran out of stock.
However during the Second World War production of nylon was switched to wartime and military uses such as parachutes so there was a shortage of nylon stockings. After the war ended, DuPont announced the return of nylon stockings.
This sparked a period of intense demand and even "nylon riots" at stores.
Women lined up in long queues outside shops, there were even reports of fights breaking out as people scrambled to get their hands on the coveted nylon stockings.
The introduction of nylon stockings by the DuPont company was one of the most successful product launches in history up to that time. The national launch in the US occurred on May 15, 1940. On that first day alone, nearly 800,000 pairs were sold across the country, selling out in most locations by noon. Within the first year; an estimated 64 million pairs were sold in the United States. Nylon quickly became so synonymous with hosiery that "nylons" became the generic term for stockings.

The Appeal: Nylons offered a superior alternative to the fragile and expensive silk stockings that dominated the market. They were more durable, sheerer, wrinkle-free and faster-drying.
The immense popularity was highlighted even further by the impact of World War II. In 1942, DuPont shifted all nylon production to the war effort to make essential items like parachutes and ropes, removing nylon stockings from the civilian market. The war time scarcity turned nylons into a highly coveted commodity, leading some women to use leg makeup or draw a seam line up the back of their legs to mimic the look. A black market also developed, selling the scarce stockings at marked-up prices. When nylon returned to the consumer market in 1945 and 1946, the demand was overwhelming. Crowds of thousands would mob department stores, leading to stampedes and chaos that were widely reported in the press as the "Nylon Riots." The consumer reaction in the 1940s demonstrated that nylon stockings were not just a successful product, but a revolutionary fashion item that women desperately desired.
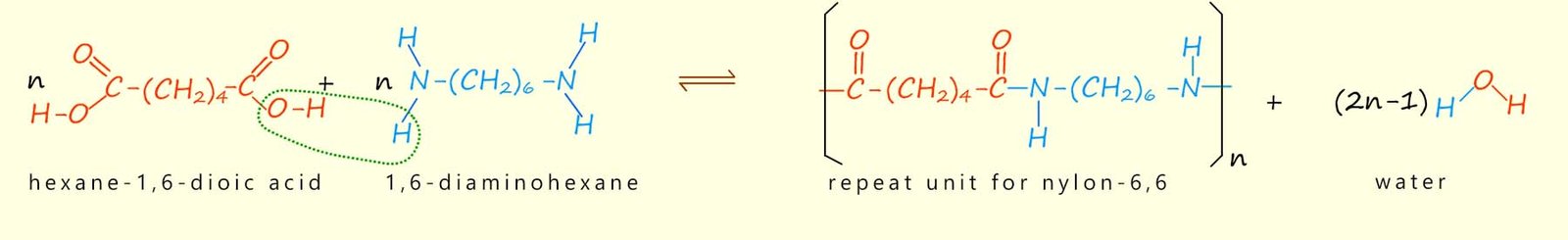
You may have seen your teacher perform the “nylon rope trick.” In this demonstration, a long thin strand of nylon is pulled from a beaker, as shown in the image below. In this reaction, a different pair of monomers is used from those mentioned above: the diamine hexane-1,6-diamine reacts with a compound called an acid chloride instead of the adipic acid, this reaction is a condensation reaction and is similar in some ways to the acid-base reaction discussed above except that it produces hydrogen chloride gas instead of water. These two monomers are used simply because it is a more efficient way of producing nylon. These two reacting monomers are immiscible; that is they behave like oil and water and do not mix. The acid chloride floats on top of the diamine and at the boundary where the two liquids meet in the beaker, it is possible to reach in with a pair of tweezers and pull out a long thin thread of nylon.

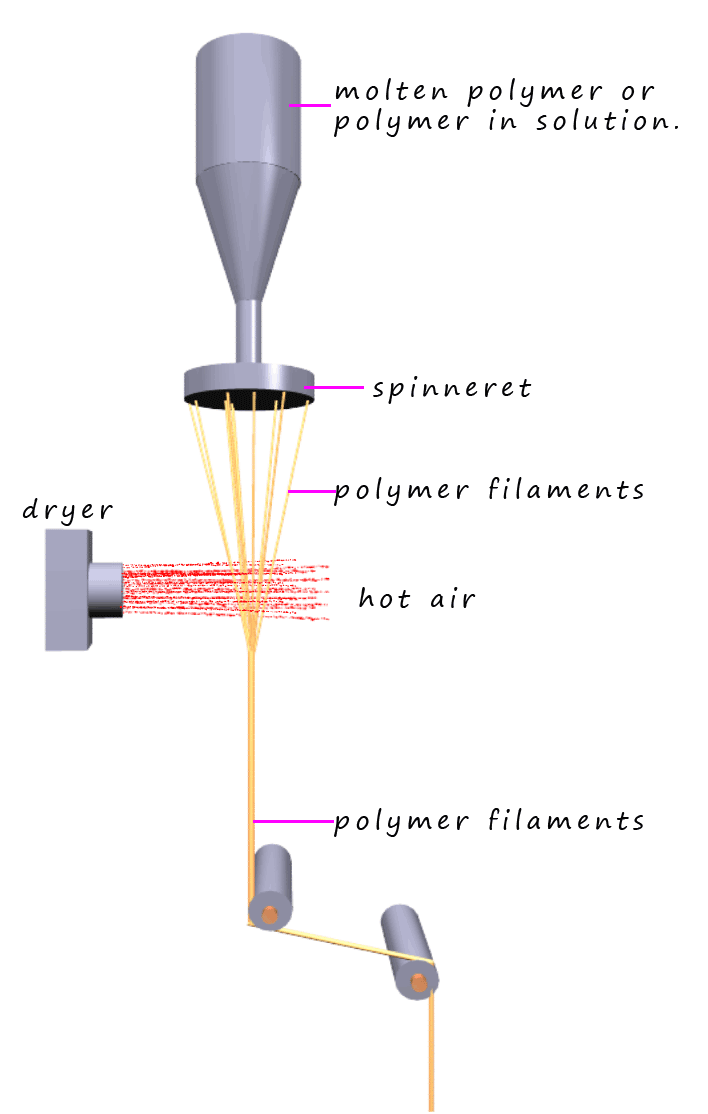
Nylon is a thermoplastic or thermosoftening polymer; this means it will soften and melt when heated. If this molten nylon is forced through a device called a spinneret (as shown in the image opposite); this simply resembles a large shower head; then long filaments of nylon thread will emerge. If these filaments are then cooled by simply being exposed to warmish air they will fully solidify and form long filaments of nylon. These can then be stretched and drawn out to form long filaments or threads which can be spun onto bobbins and the filaments or threads can then be used to make fabrics or other items as necessary. Drawing or stretching the nylon filaments results in the formation of long parallel polymer chains which will pack closely together which enables them to form lots of intermolecular bonds to neighbouring polymer chains; this intermolecular bonding between the polymer chains dramatically increases the strength of the nylon filaments formed.
Nylon being a thermoplastic means that it can be melted to form a viscous fluid which flows well and can be shaped and moulded into various objects such as pipes, tubes, films, screws as well as nuts and bolts. The fact that it is easily shaped gives it many industrial and commercial uses in such items as moulded machine parts to simple washer or gears and bearings in machinery, appliances and even children's toys. The bristles in your toothbrush are also likely to contain nylon fibres. Nylon is also mixed with other natural fibres to increase their wear resistance and usability e.g. nylon is mixed with wool to make carpets.
The montage below shows just a few of the many uses of nylon from guitar strings, to ropes, raw plugs and wind socks.

Select the correct monomers from the activity below to produce the polyamide nylon-6,6. Press the check answer button when you're done.
Tap a monomer card, then tap a target box to place it. Fill Monomer A (diacid), Monomer B (diamine), and the By-product box.
n H₂N–(CH₂)₆–NH₂ + n HOOC–(CH₂)₄–COOH → [–NH–(CH₂)₆–NH–CO–(CH₂)₄–CO–]ₙ + 2n H₂O
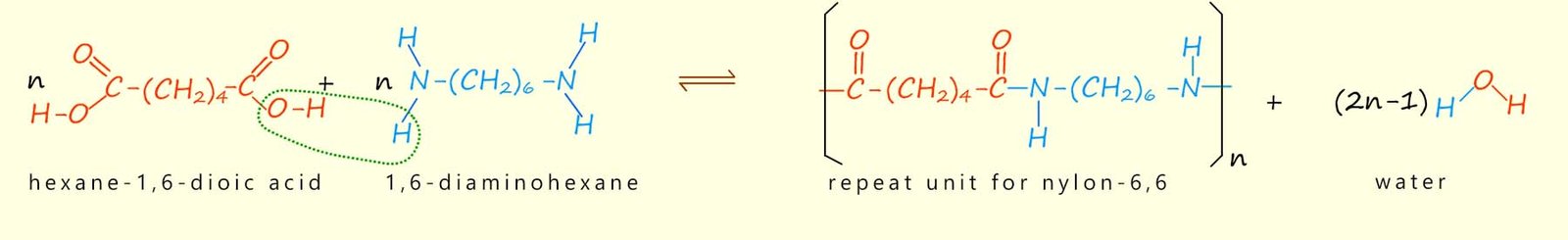
This is a condensation polymerisation: amide bonds form and water is released.
Amino acids contain the acidic carboxyl group (-COOH) and the basic amino group (-NH2) in one molecule. So instead of using two separate monomers; one with the acidic carboxyl group and one with the basic amino group to make a polyamide as saw above why not just use a single monomer with both these reactive functional groups on the ends of each monomer?
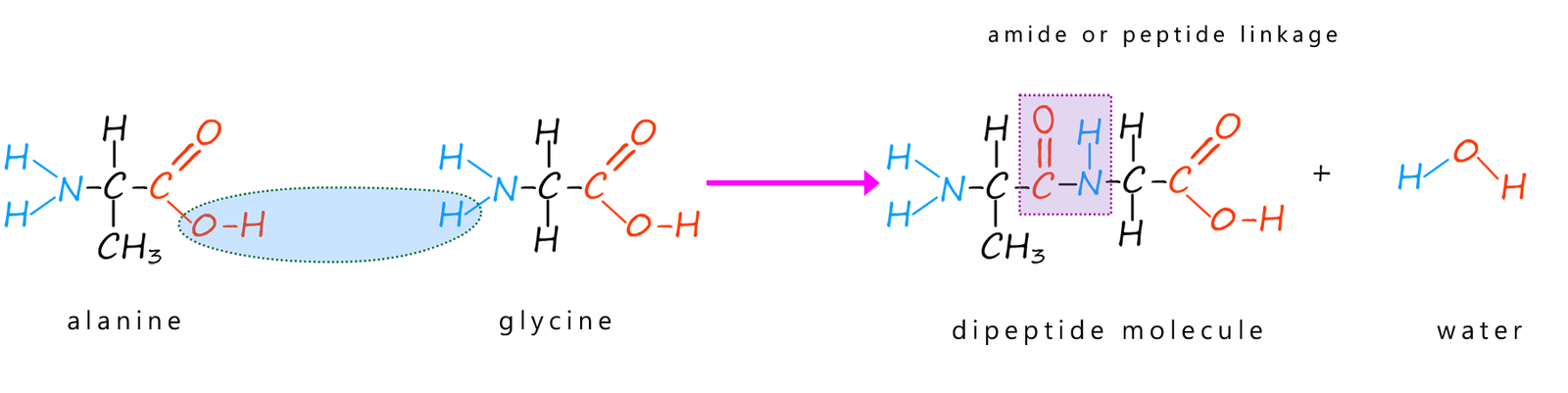
Well in the case of amino acids that is exactly what happens when they form polypeptides and proteins; for example the amino acids alanine and glycine can undergo a condensation reaction to form a dipeptide molecule as shown below. This dipeptide molecule contains an amide or peptide bond. However the dipeptide molecule still has reactive amino (-NH2) and carboxyl groups (-COOH) on the ends of the molecule and can readily undergo more condensation reactions to form a polypeptide molecule or a protein.
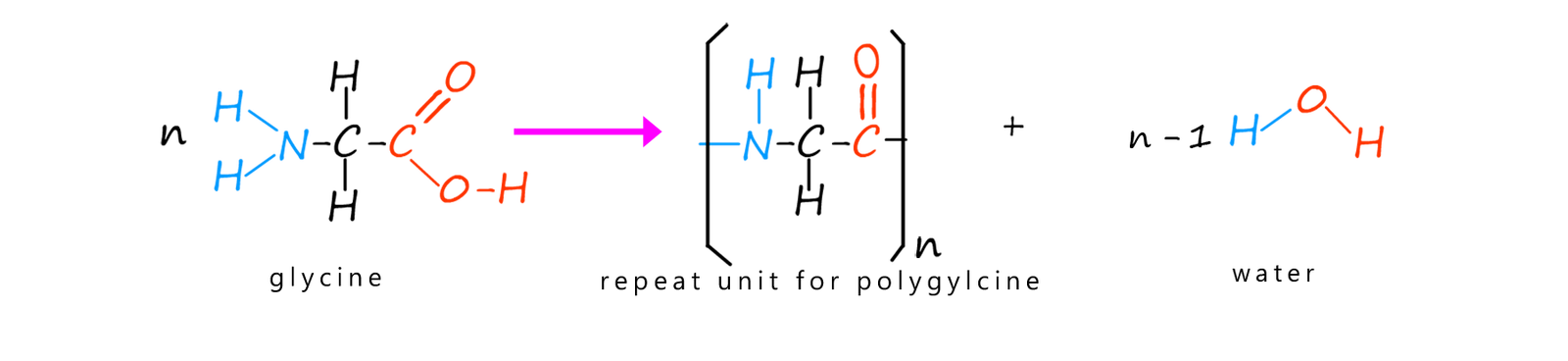
It is not even necessary to start with different amino acids. Heating a single amino acid monomer will result in the formation of a polyamide. We can show this simply as follows where the amino acid glycine can polymerise to form the polymer polyglycine when heated.
Use the time-line buttons below for a brief history of the early "nylon story".
Wallace Carothers and his team at DuPont develop the first successful polyamide: nylon. The key idea is condensation polymerisation forming amide links.


Nylon stockings went on sale and millions of pairs sold in hours.


Nylon production shifted to military uses such as parachutes and cords.


After the war, crowds rushed to buy stockings when nylon returned to stores.


–CO–NH– (the amide or peptide linkage).