

Chemistry only
Carboxylic acids are another example of a homologous series of organic compounds. Carboxylic acids are a family of weak
acids which contain the carboxyl group (-COOH) functional group. You may have met some of these carboxylic acids before and not realised it at the time; for example ant bites and wasp stings contain the simplest carboxylic acid; methanoic acid while ethanoic acid is the main ingredient in vinegar. Ethanoic acid is also used to marinated meat and fish and to pickle vegetables.
However as well as common uses mentioned above the carboxylic acids have many industrial uses and they are used in the manufacture of many important industrial and biological chemicals e.g. they are used in the manufacture of solvents, adhesives, soaps, rubber, dyes, perfumes and many types of drugs.
| alkane | carboxylic acid |
|---|---|
| methane | methanoic acid |
| ethane | ethanoic acid |
| propane | propanoic acid |
| butane | butanoic acid |
Ball and stick models as well as the structural formula and displayed formula for the first 4 carboxylic acids are shown below
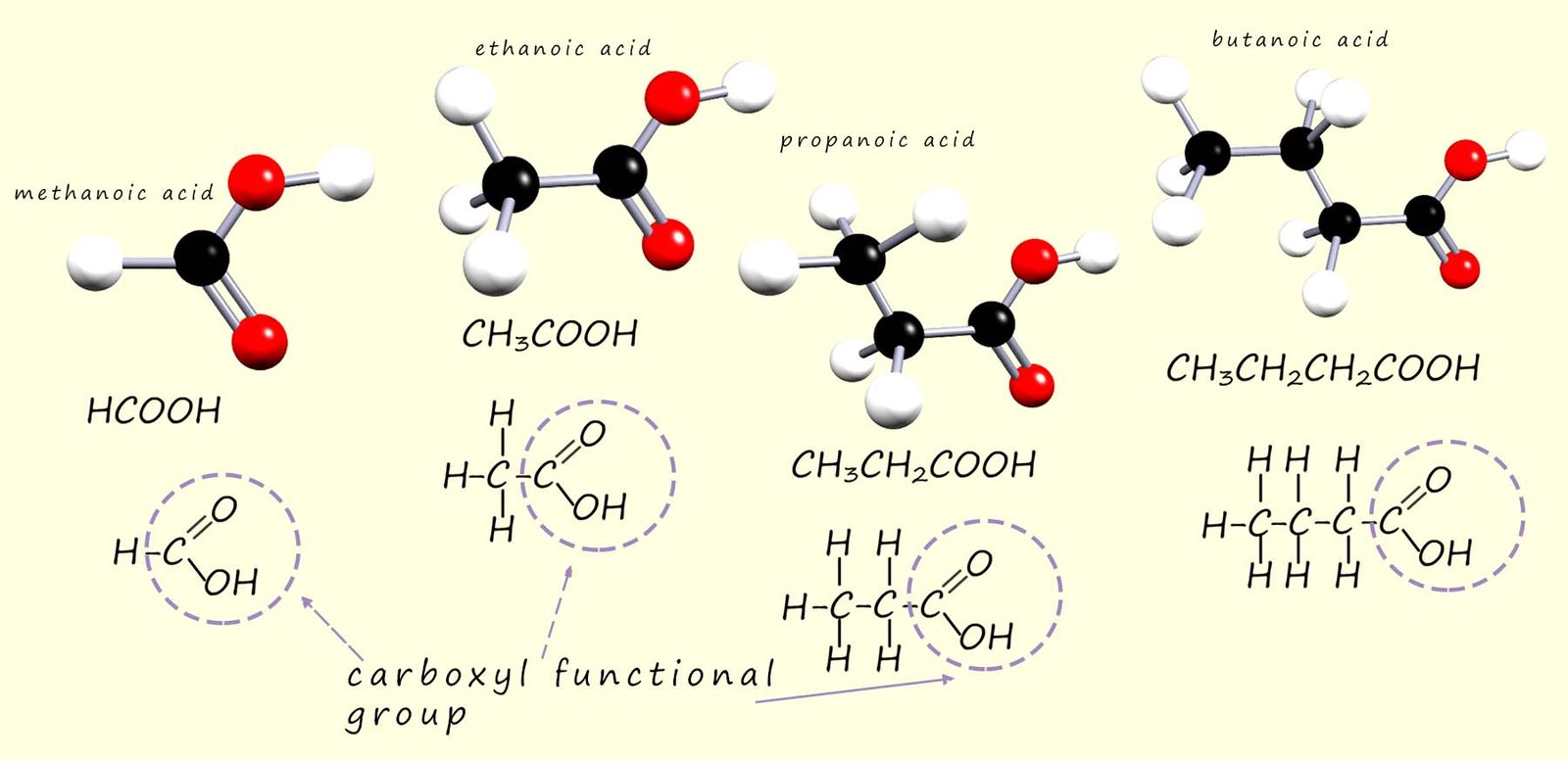
As before when we draw out the formula for these compounds we always draw them with the carboxyl functional group shown e.g. methanoic acid is HCOOH and never CO2H. Ethanoic acid is CH3COOH and never C2H402.
Carboxylic acids with less than 6 carbon atoms are
soluble in water; they dissolve to form
weak acids.
If you remember a weak acid forms when a solid or liquid acidic compound only partly
ionises when it is added
to water.
The dissociation of a carboxylic acid in
water is shown
below. Here the carboxylic acid molecule ionises to form a carboxylate anion (RCOO-) and a
hydrogen ion (H+).
It is the presence of the hydrogen ion (H+) which gives the molecule
its acidic properties. However very
few of the carboxylic acid molecules actually ionise, most remain intact.
This means that the position of equilibrium lies
very much to the left hand side; that is the reactants; hence the reason why carboxylic
acids are weak acids with fairly high
pH values of between 3-5.

As an example consider the simplest carboxylic acid; methanoic acid (HCOOH). When methanoic acid is added to water only a small proportion of the methanoic acid molecules will ionise to form methanoate ions and hydrogen ions. Most of the methanoic acid molecules stay intact and do not dissociate or break down in water, we can show this as:

similarly with ethanoic acid we have:

In the symbolic equations below the water has been omitted to simplify the equations, the equations show what happens when a carboxylic acid molecule is added to water:
| carboxylic acid | molecular formula | ion formed | structural formula of ion |
|---|---|---|---|
| methanoic | HCOOH | methanoate | HCOO- |
| ethanoic | CH3COOH | ethanoate | CH3COO- |
| propanoic | C2H5COOH | propanoote | C2H5COO- |
| butanoic | C3H7COOH | butanoate | C3H7COO- |

A reaction you have seen before is when hydrochloric acid reacts with a metal carbonate such as sodium carbonate or calcium carbonate to form a salt, water and carbon dioxide gas. Equations for these reactions are shown below:
swapping the strong acid hydrochloric acid for a weak carboxylic acid will produce a similar reaction but it will be much slower e.g.
Example 3:The apparatus to compare the rate of reaction of a weak carboxylic acid and a strong acid with chalk (calcium carbonate) is shown below. Using this simple set-up we can compare the rate of reaction of a strong and a weak acid with calcium carbonate (chalk). All we have to do is count the number of bubbles or modify the apparatus to include a gas syringe and measure the volume of carbon dioxide gas released in a given time.
Use the flashcards below to check your understanding on the structure, reactions and properties of the carboxylic acids.














