


A condensation reaction is one where small reactive molecules join together to make a larger molecule and at the same time a small molecule such as water (H2O), methanol (CH3OH) or hydrogen chloride (HCl) gas is released or eliminated.
Condensation polymers or step growth polymers are large molecules which are built up by joining together small monomer molecules. You will have already met many examples of natural condensation polymers in chemistry already though perhaps you may never have realised it at the time. Carbohydrate molecules such as starch and cellulose are examples of natural condensation polymers made joining together thousands of smaller glucose molecules together. Proteins are another example of a condensation polymer made by joining together lots of small amino acid monomers together. Finally another condensation polymer you will meet in your studies is DNA, this is a polymer formed by joining together lots of small monomers called nucleotides.
These examples of condensation polymers are all naturally occurring polymers and in this case the small molecule which is lost is always water, however with synthetic condensation polymers, especially if the reaction involves equilibrium it maybe more suitable to have small molecules such as hydrogen chloride gas be eliminated since removal of this gas will push the equilibrium in favour of the products and so increase the reaction yield..
Polyesters are condensation polymers formed by the condensation reaction of a dicarboxylic acid and a diol. You are not doubt familiar with the reaction of a carboxylic acid with an alcohol to form an ester linkage, this is outlined below:
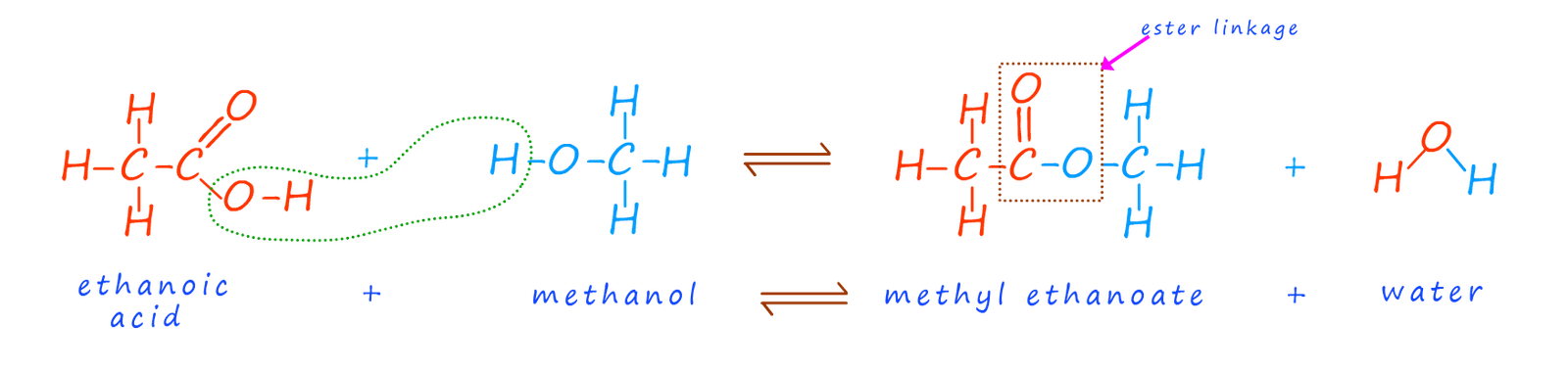
The ester linkage is formed by the condensation reaction of the hydroxyl group (-OH) on the carboxylic acid with a hydrogen atom from the hydroxyl group on the alcohol. The problem is that once these two reactive parts on each of the functional groups have reacted together no further reactions are possible. To make a condensation polymer such as polyester what is needed is a dicarboxylic acid and a diol. That is a carboxylic acid molecule with two carboxyl groups (-COOH) and an alcohol with two hydroxyl (-OH) functional groups on each end of the molecule. The table below shows the first two members of the diol homologous series of alcohols, here you can see that each member of this homologous series has two hydroxyl functional groups (O-H) on each end of the molecule.
| diol | displayed formula | formula |
|---|---|---|
| ethane-1,2-diol | 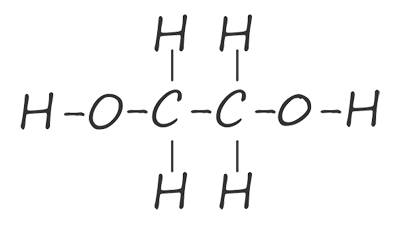 |
HOCH2CH2OH |
| propane-1,3-diol | 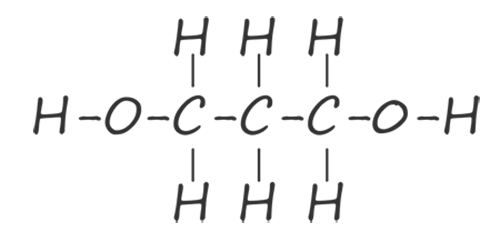 |
HOCH2CH2CH2OH |
Dicarboxylic acids like diols have two functional groups on each molecule; in the case of dicarboxylic acids each acid molecule has two carboxyl functional groups (COOH) per molecule. The first two members of this diacid homologous series are shown in the table below:
| carboxylic acid | displayed formula | formula |
|---|---|---|
| ethane-1,2-dioic acid |  |
HOOCCOOH |
| propane-1,3-dioic acid | 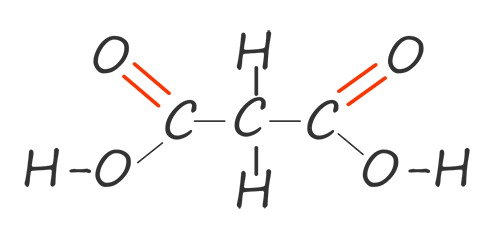 |
HOOCCH2COOH |
Having reactive functional groups on each end of the molecule would mean that when these two molecules react to form an ester linkage there would still be unreacted carboxyl and hydroxyl functional groups on the end of each of the molecules to continue the reaction, this is outlined in the image below:
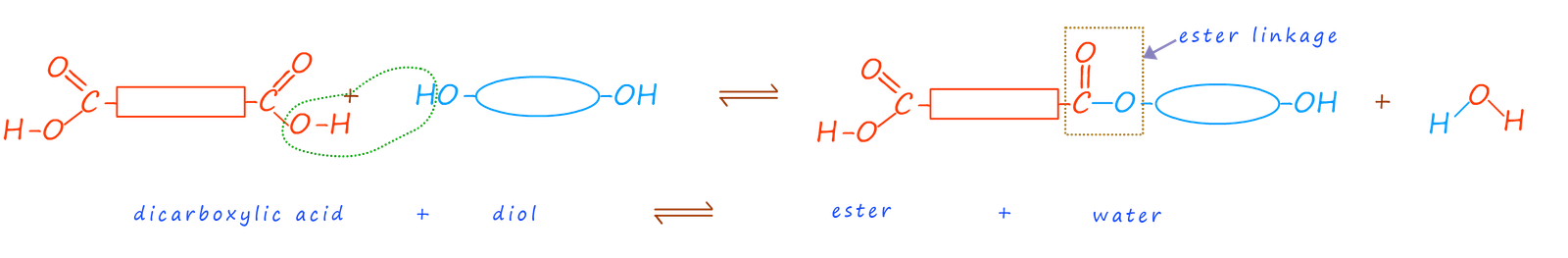
In this example once the new ester molecule forms there is still a carboxyl group from the carboxylic acid and a hydroxyl group from the alcohol on the end of the ester molecule. Each of these functional groups can now react with another dicarboxylic acid and another diol molecule and in this way slowly build up the length of the polymer chain. Eventually there would be lots of ester linkages in the molecule formed; that is it is a polyester.
The polyester molecule is made up of many identical repeating units; much like the beads in a pearl necklace. In the case of a polyester the repeat unit is formed by the loss of the -OH group from the dicarboxylic acid and a -H atom from the hydroxyl group on the diol. Recall that the esterification reaction is a condensation reaction in which a small molecule; in this case water is eliminated. For every n molecules of diacid and diol that react (2n-1) molecules of water are eliminated. The repeat unit for a typical polyester is shown below:
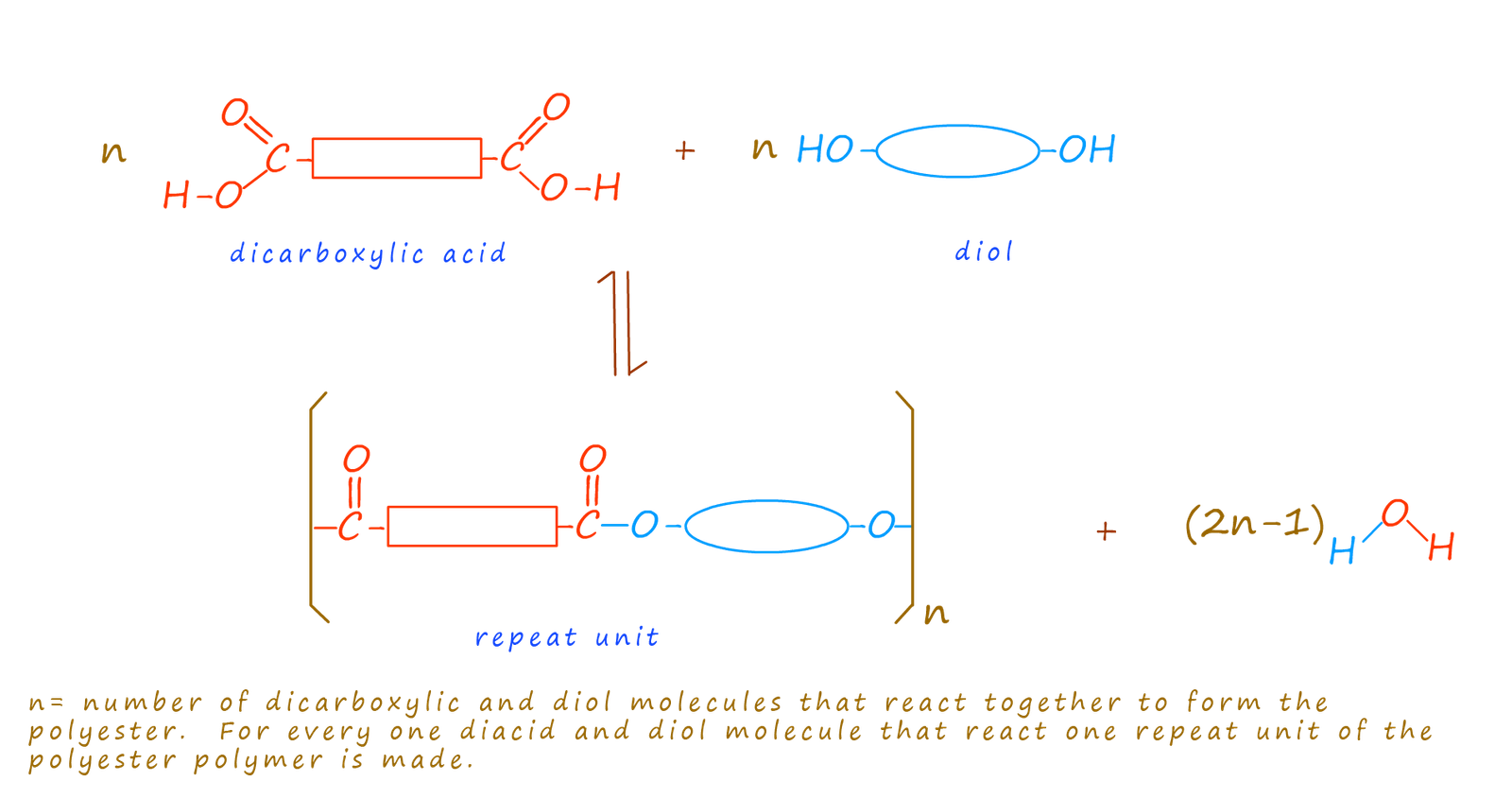
There a numerous ways to form polyester; perhaps the one you are most familiar with is the one mention above; that is using a dicarboxylic acid and an diol:
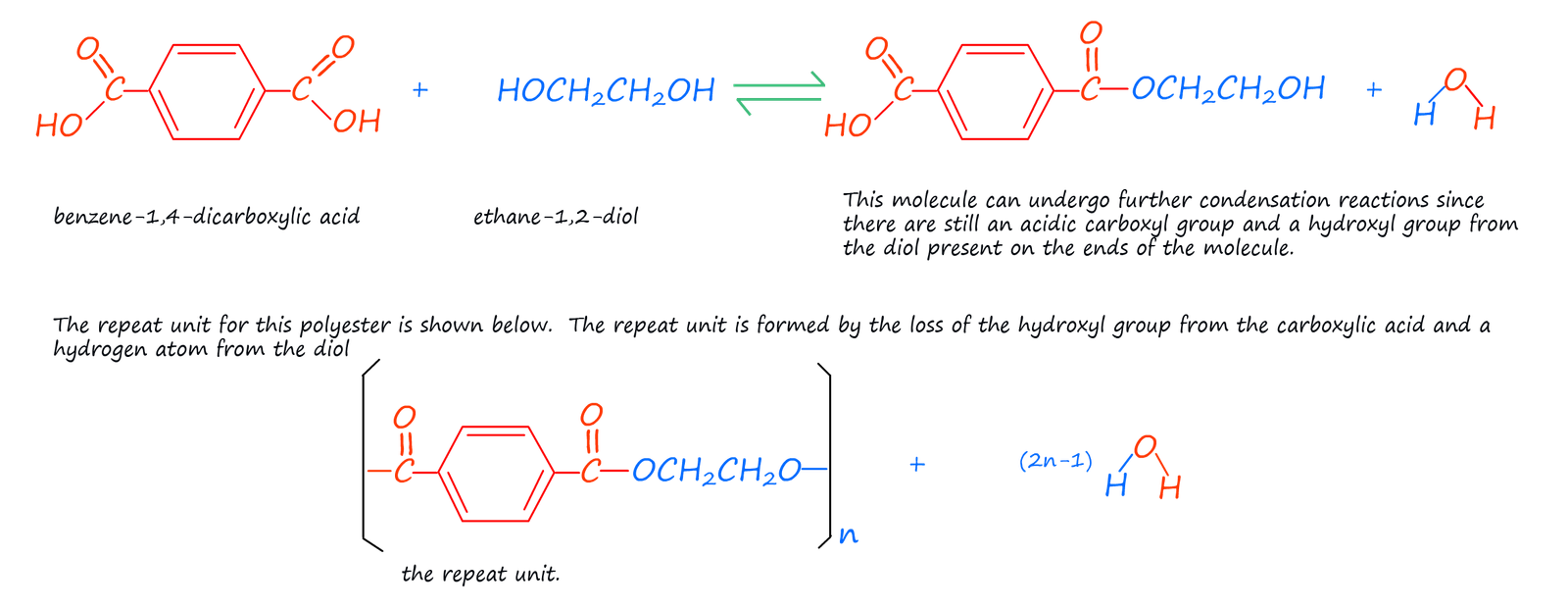

Terylene is not a new material, it was first made in the 1940s by British chemists. It was often made into a fibre and mixed with other naturally occurring polymers such as wool or cotton. The properties of Terylene complemented those of many natural fibres well; whereas natural materials such as cotton and wool absorb large amounts of water and are not particularly hard wearing Terylene resist absorbing moisture and also its ability to resist wrinkles and creasing and the fact that it is a hard wearing, long lasting and inexpensive material made it an ideal choice for use in clothing, particularly when mixed with cotton.
Terylene does have some disadvantages though; it is not a breathable material and it does not cope well with high temperatures which means that when it is mixed with cotton and wool in clothing care needs to be taken in how it is dried and ironed. Being a synthetic polymer or plastic means that it has a artificial feel and texture, but this can be overcome to some extent when mixed with a natural fibre. Terylene can be readily made into fibres and mixed or blended with natural materials such as wool and cotton to make a wide variety of clothing items such as: shirts, jackets, socks, ties, trousers, curtains and even carpets; where it was originally sold under the name of Dacron in the USA. Terylene fibres being strong, hard wearing and water resistant make it suitable for use in such items as ropes, shower curtains and raincoats.
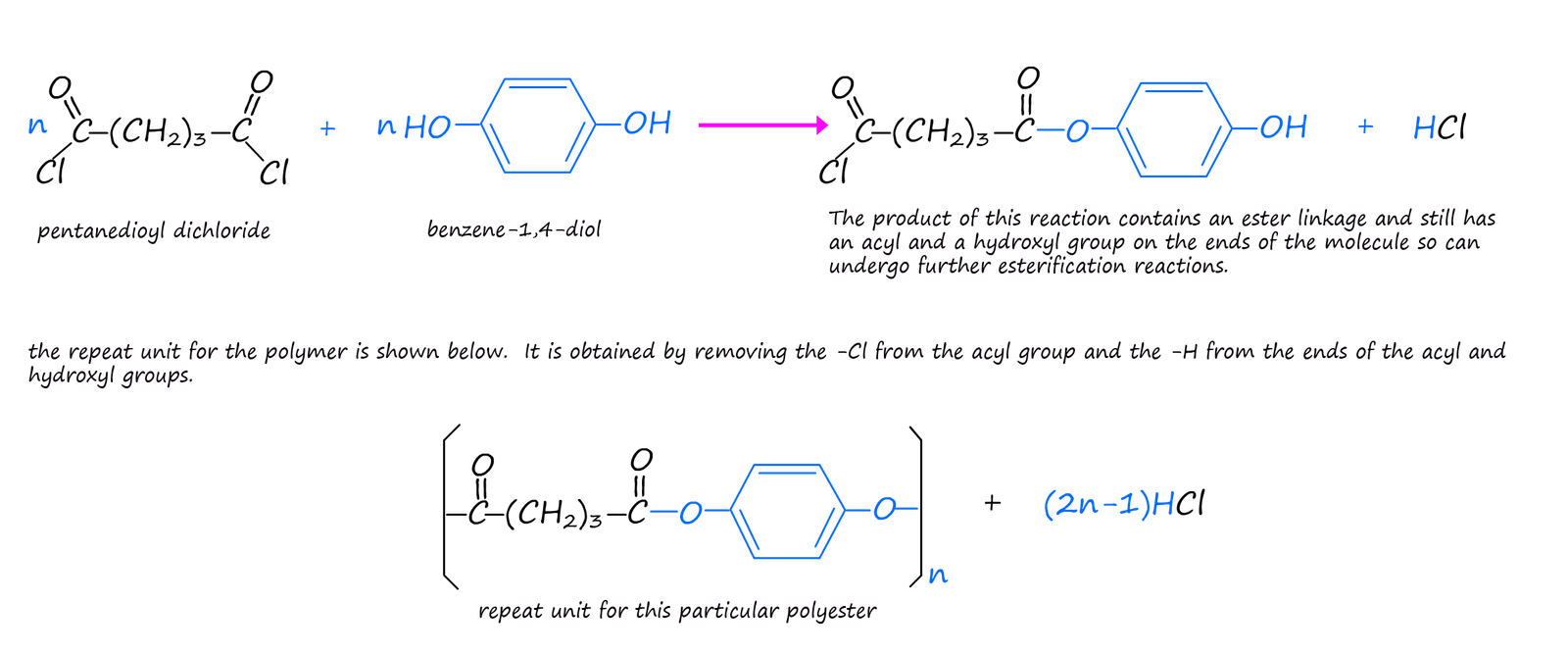
However you may also recall that another way of forming an ester is by the reaction of an acyl chloride with an alcohol:
The examples of condensation polymers given above use two different monomers with each monomer having the same two functional groups on the ends of the monomer molecules. This is the usual case but it by no means the only option; it is possible to have monomers with two different functional groups on the ends of the monomer molecule or even have one single monomer with different functional groups on its ends. Lactic acid is one such monomer. The structure of lactic acid (2-hydroxypropanoic acid) is shown below. It contains the acidic carboxyl group (-COOH) on one end of the molecule and a hydroxyl group (-OH) on the other end. Lactic acid molecules can undergo a condensation polymerisation reaction to form the polyester poly(lactic acid) or PLA as shown below.
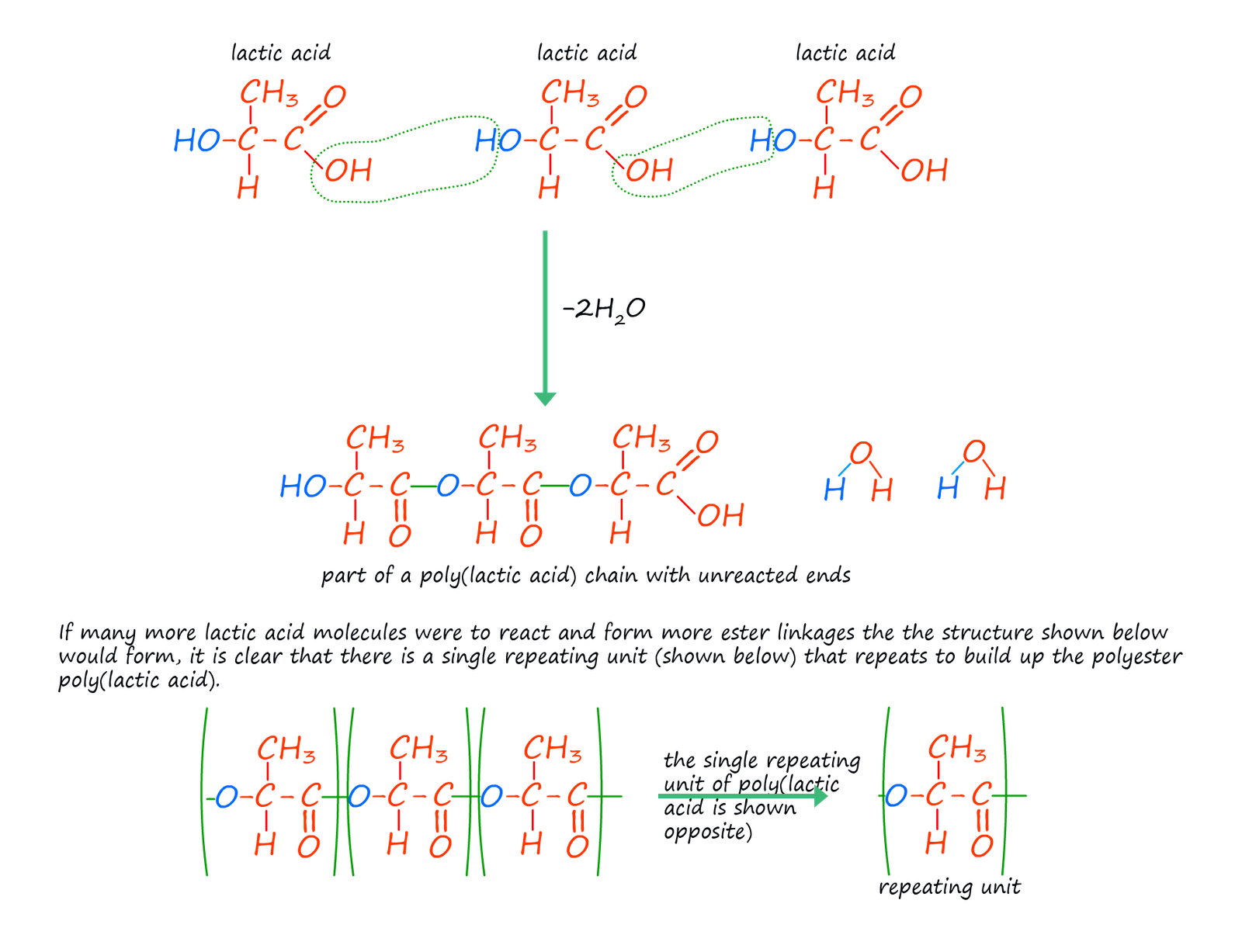

Poly(lactic acid) is a biodegradable polyester since lactic acid is a natural occurring substance which is usually obtained from either maize or corn; this has lead to an increase in possible applications for its use which may result in a reduction in the large amount of non-biodegradable plastics that go to landfill. The main uses for PLA include: