

Acid halides are a derivative of carboxylic acids where the -OH group in the carboxyl group (-COOH) present in a carboxylic acid is replaced by a halogen (F, Cl, Br or I). Most acid chlorides are volatile liquids at room temperature that react violently with water, though benzoyl chloride (which contains an aromatic ring) only hydrolyses slowly with water. Most acid chlorides may react violently with water but they are soluble in many organic solvents such as tetrahydrofuran or dichloromethane.
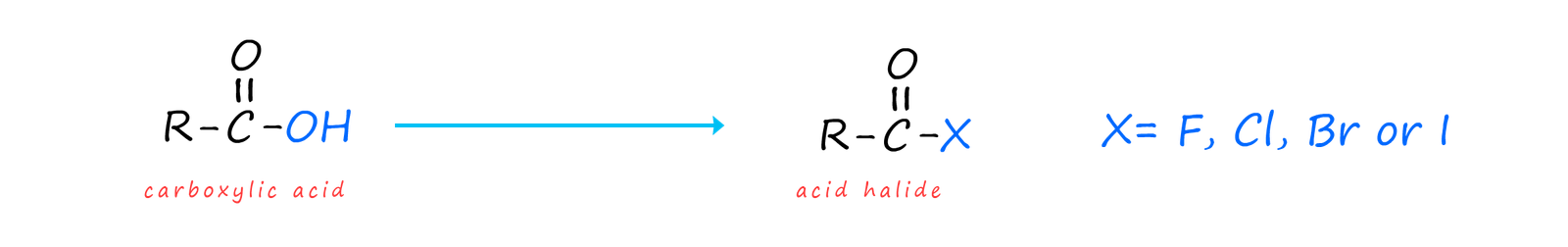
However the only acid halides you are likely to ever have to deal with are acid chlorides. Acid chlorides are named from the parent carboxylic acid by simply changing the -ic in the carboxylic acid name with -yl e.g.
| carboxylic acid | acid chloride | structure of acid chloride |
|---|---|---|
| ethanoic acid | ethanoyl chloride |  |
| propanoic acid | propanoyl chloride |  |
| butanoic acid | butanoyl chloride |  |
| benzoic acid | benzoyl chloride |  |
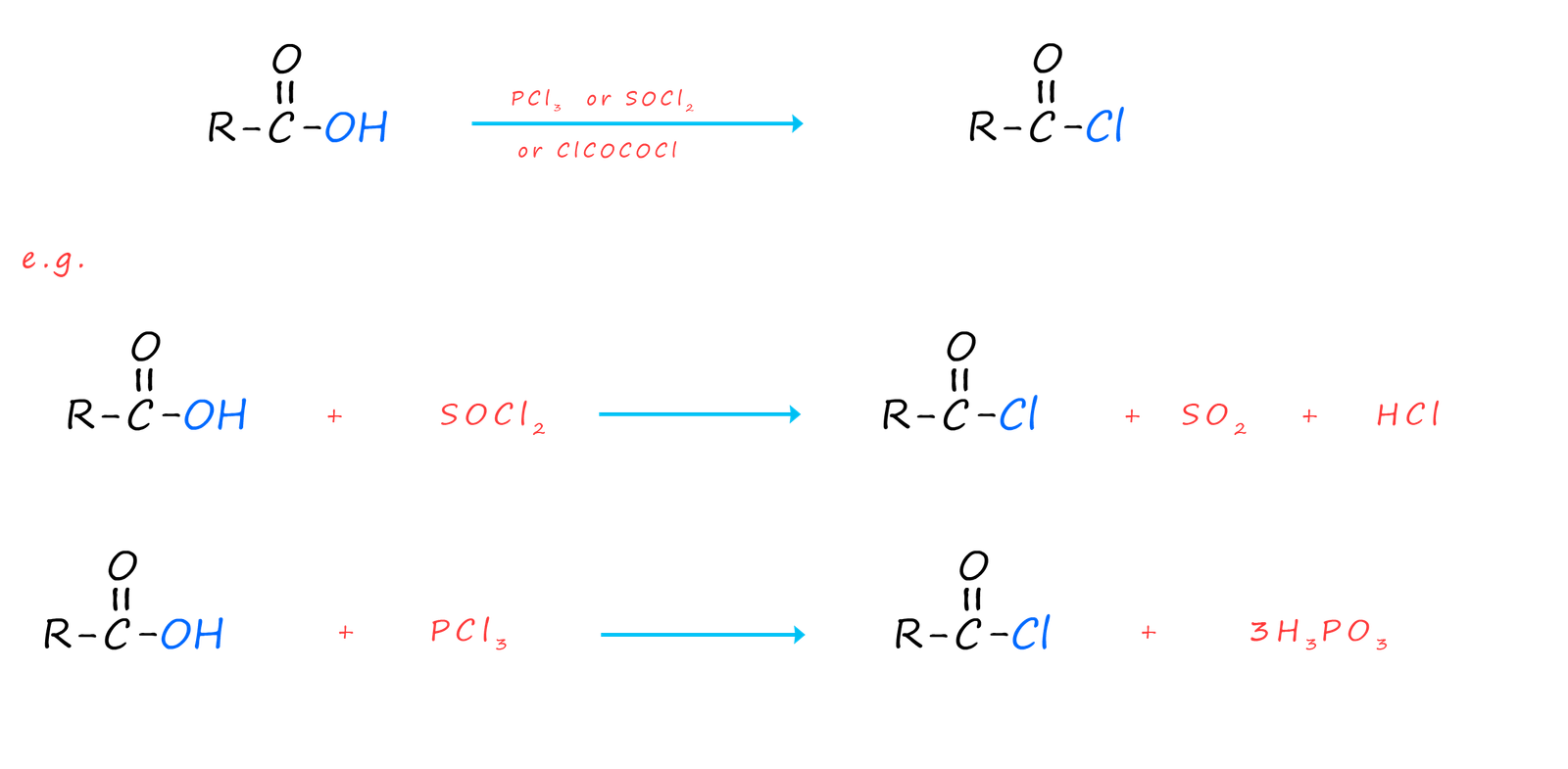
Acid chlorides can be prepared directly from their parent carboxylic acid by reaction with a suitable chlorinating agent such as thionyl chloride (SOCl2), phosphorus trichloride (PCl3) or oxalyl chloride (ClCOCOCl), equations for these chlorination reactions are shown below:
Acid halides are the most reactive of all the carboxylic acid derivatives. The carbon atom in the carboxyl group being δ+ is readily attacked by nucleophiles and since the attached halogen atom is able to form a stable anion it is a good leaving group. The mechanism of this reaction is outlined below, shows a typical addition-elimination reaction of an acid halide with a nucleophile (Nu).
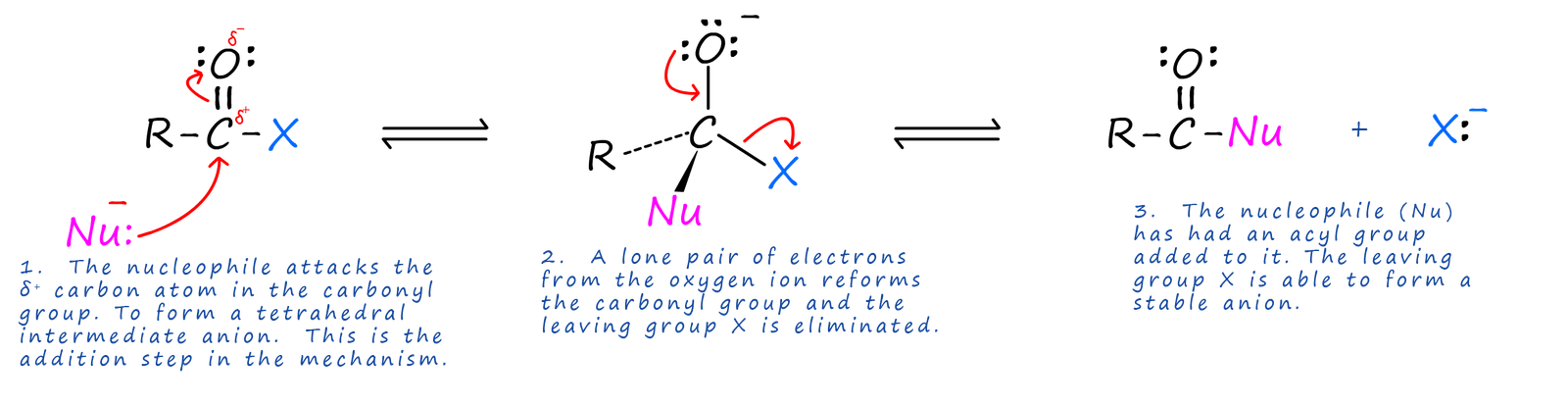
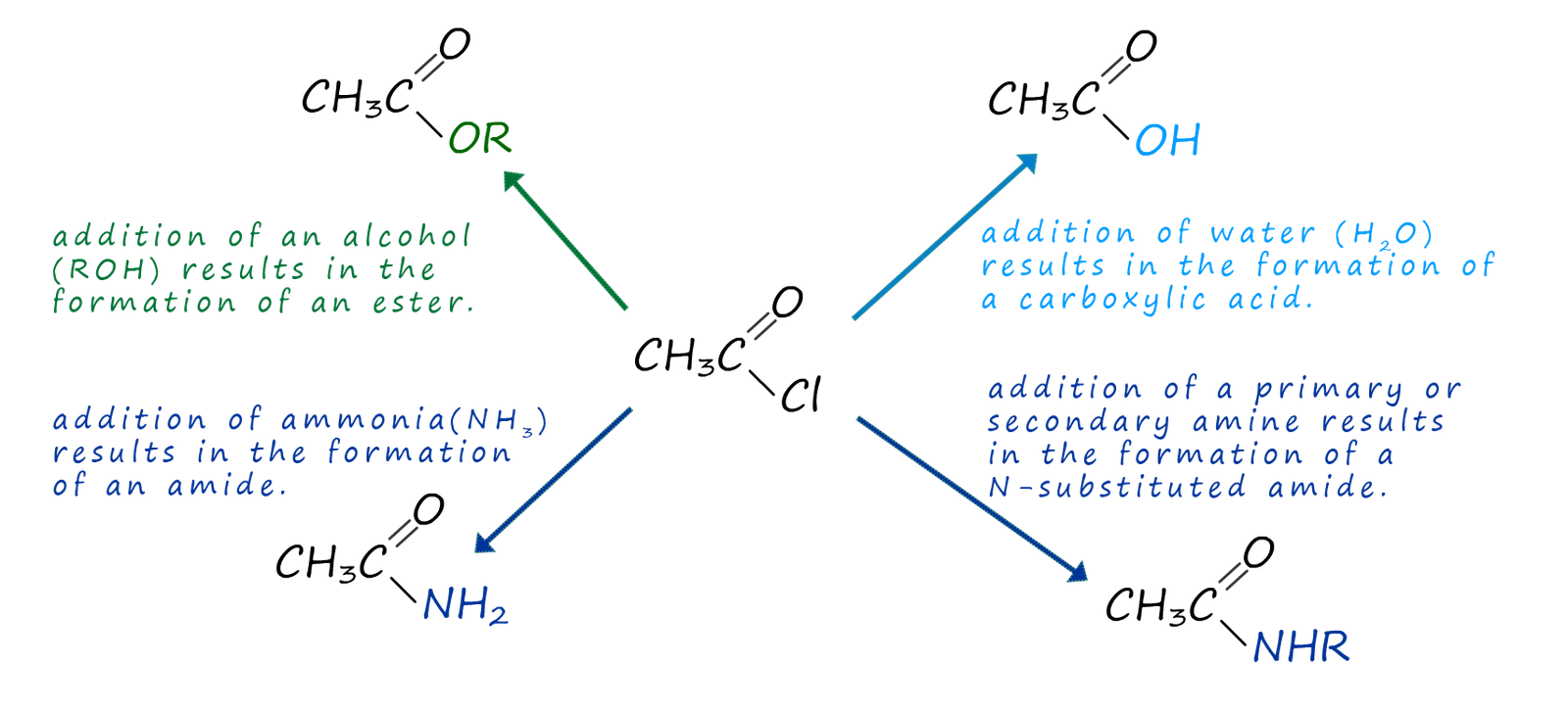
The end result of the above reaction is that an acyl group (RCO) has been added to the nucleophile. One of the main uses of acid chlorides and also acid anhydrides is as acylating agents, that is they will add an acyl group (RCO) to another species. These acylation reactions can used to produce a wide range of useful and valuable substance, as shown in the image below:
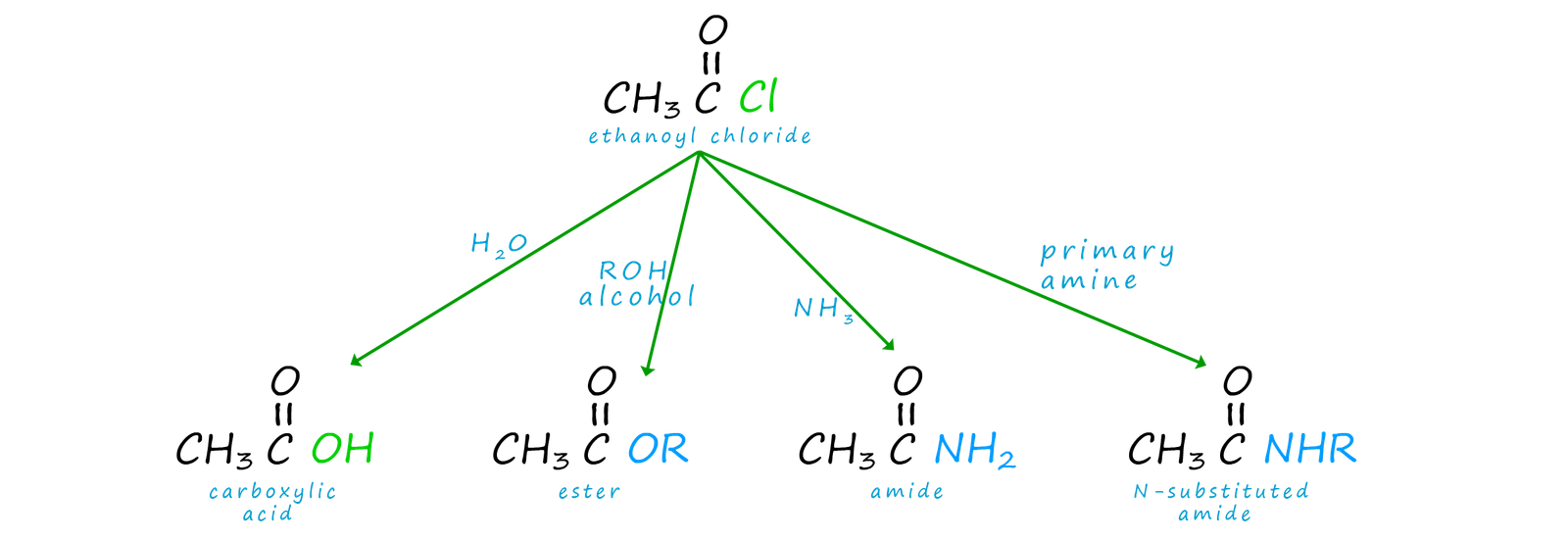
Acid chlorides react violently with water to form carboxylic acids and release hydrogen chloride gas. Ethanoyl chloride is perhaps the acid chloride you are mostly likely to meet and use in the chemistry lab. It is a colourless liquid; now if the top of a bottle of ethanoyl chloride is removed in the lab then white choking fumes of hydrogen chloride gas will be formed at the mouth of the bottle. Hydrogen chloride is a colourless gas but it reacts with the moisture present in air to form white mist of hydrochloric acid droplets in the air. If a bottle of concentrated ammonia is held close to the open bottle of ethanoyl chloride then a dense white cloud of ammonium chloride forms. The reaction of water with ethanoyl chloride can be described as a hydrolysis reaction and an outline of the mechanism is shown below.

Acid chlorides react with alcohols to form esters and hydrogen chloride gas. The reaction of alcohols with other organic compounds is often called alcoholysis. The reaction of alcohols with acid chlorides is an excellent method of preparing esters and good yields are easily obtainable since this avoids the equilibrium reaction of a carboxylic acid/alcohol esterification reaction. The reaction is usually carried out in the presence of an amine base which can react with the acidic HCl gas produced and prevent it from carrying out any unwanted side reactions. The mechanism of this reaction is very similar to that of the hydrolysis reaction using water, but that is to be expected. If picture a water molecule as H-O-H and an alcohol molecule as R-O-H then it is easy to see why these two mechanism are similar.
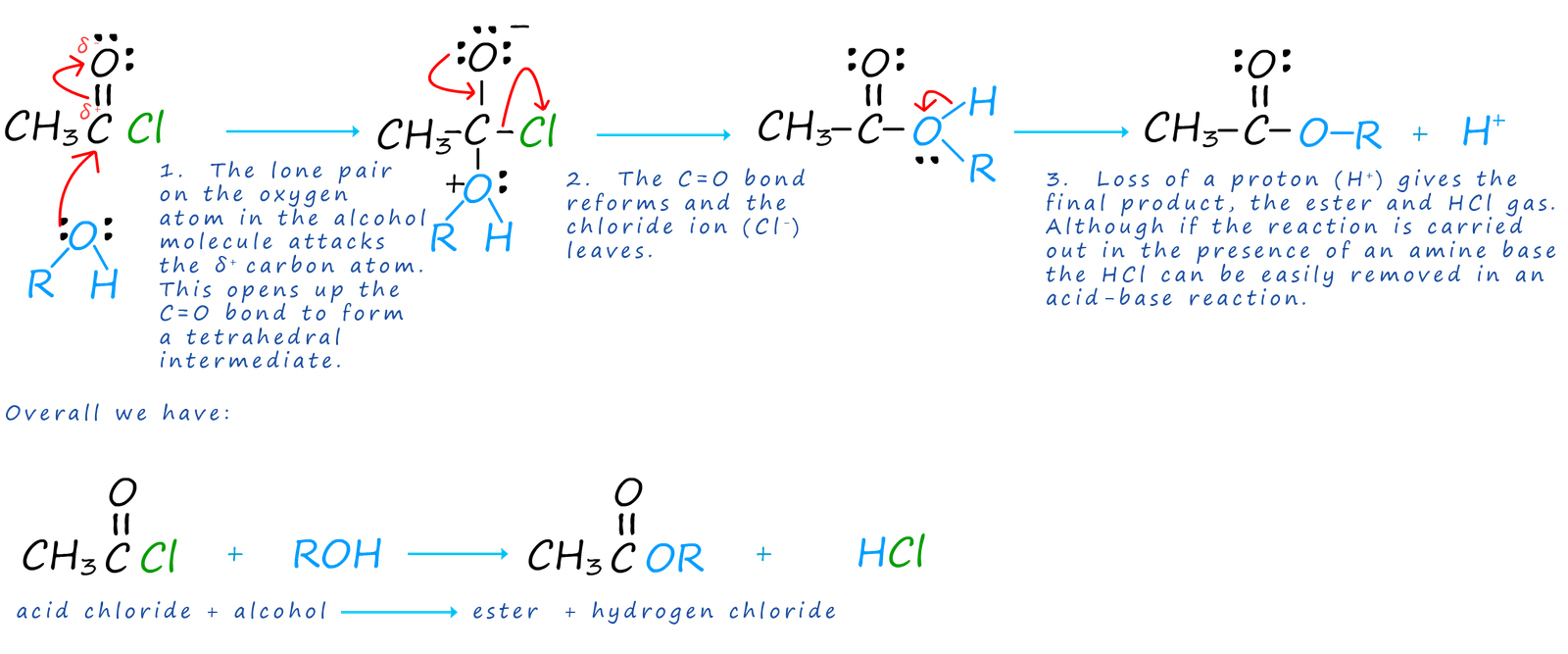
We are perhaps used to seeing ammonia and amines used as bases, however good bases are also generally good nucleophiles. Primary and secondary amines and ammonia readily react with acid chlorides in addition-elimination reactions similar to that for water and alcohols. However the product of the reaction this time is an amide if ammonia is used as the attacking nucleophile, while a N-substituted amide will be formed if we use a primary or secondary amine as the attacking nucleophile.

We can summarise the reactions of acid chlorides as follows:
Nucleophilic addition-elimination involves: