

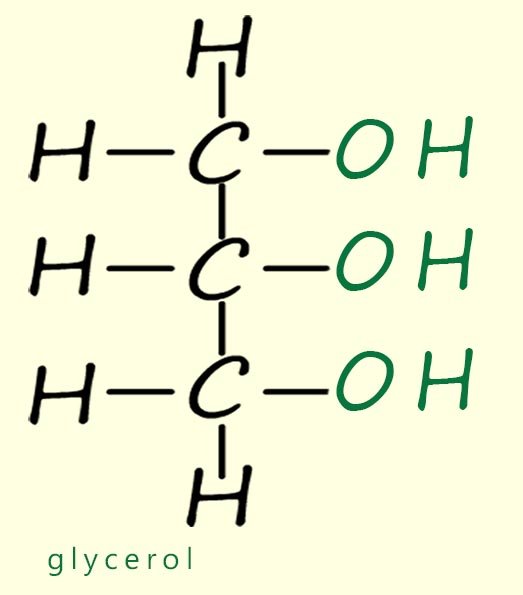
Lipids are a group of naturally occurring organic molecules that are found in almost all living organisms. Chemically lipids are esters formed in a condensation reaction between a long chain carboxylic acid and an alcohol; usually an alcohol molecule called glycerol (shown in the image opposite). In chemistry when we talk about lipids we are usually talking about the molecules that are found in vegetable oils and animal fats. Common examples of vegetable oils include olive oil, sunflower oil and rapeseed oil, while examples of animal fats include butter, lard and tallow.
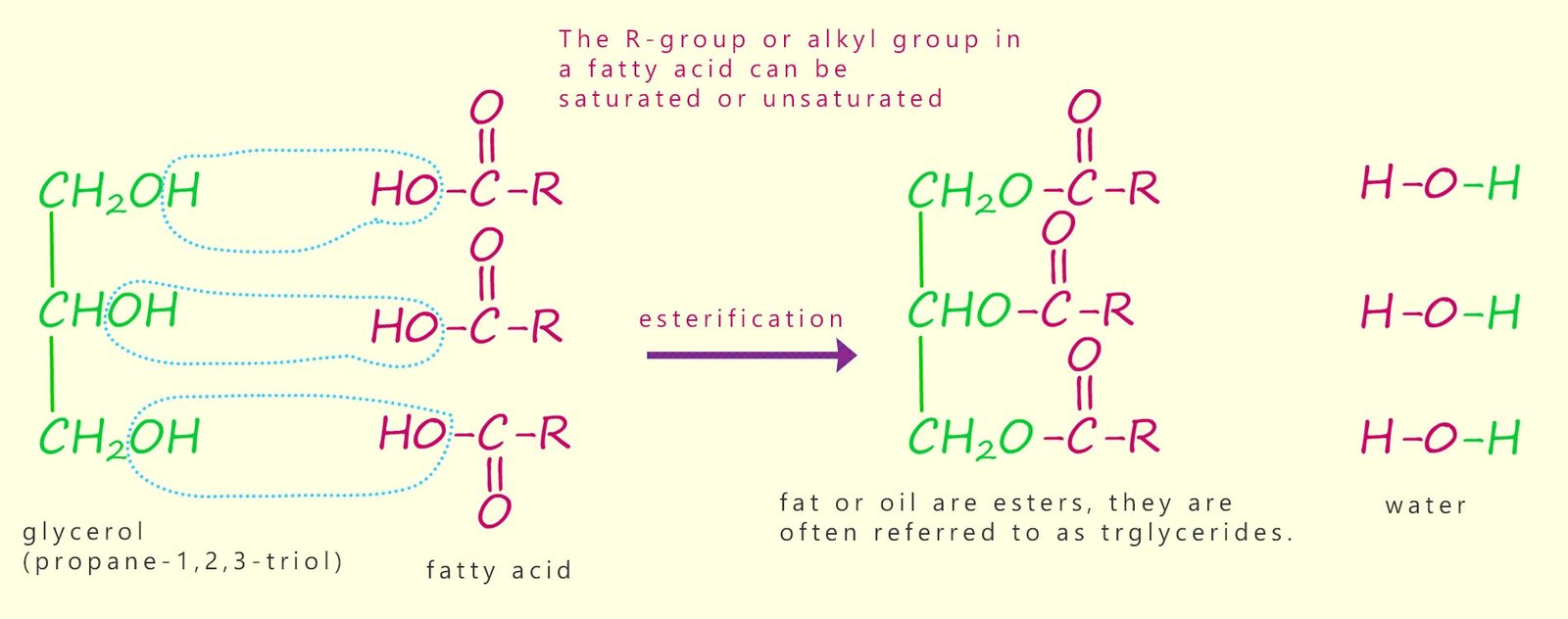
Oils and fats are esters; more specifically they are triesters formed when one molecule of glycerol; an alcohol with three hydroxyl functional groups reacts with long-chain carboxylic acids known as fatty acids. These long-chain fatty acids consist of long hydrocarbon chains with a carboxylic acid carboxyl group (-COOH) at one end. These long chain fatty acids can be saturated, unsaturated, or polyunsaturated, depending on whether carbon-carbon double bonds are present.
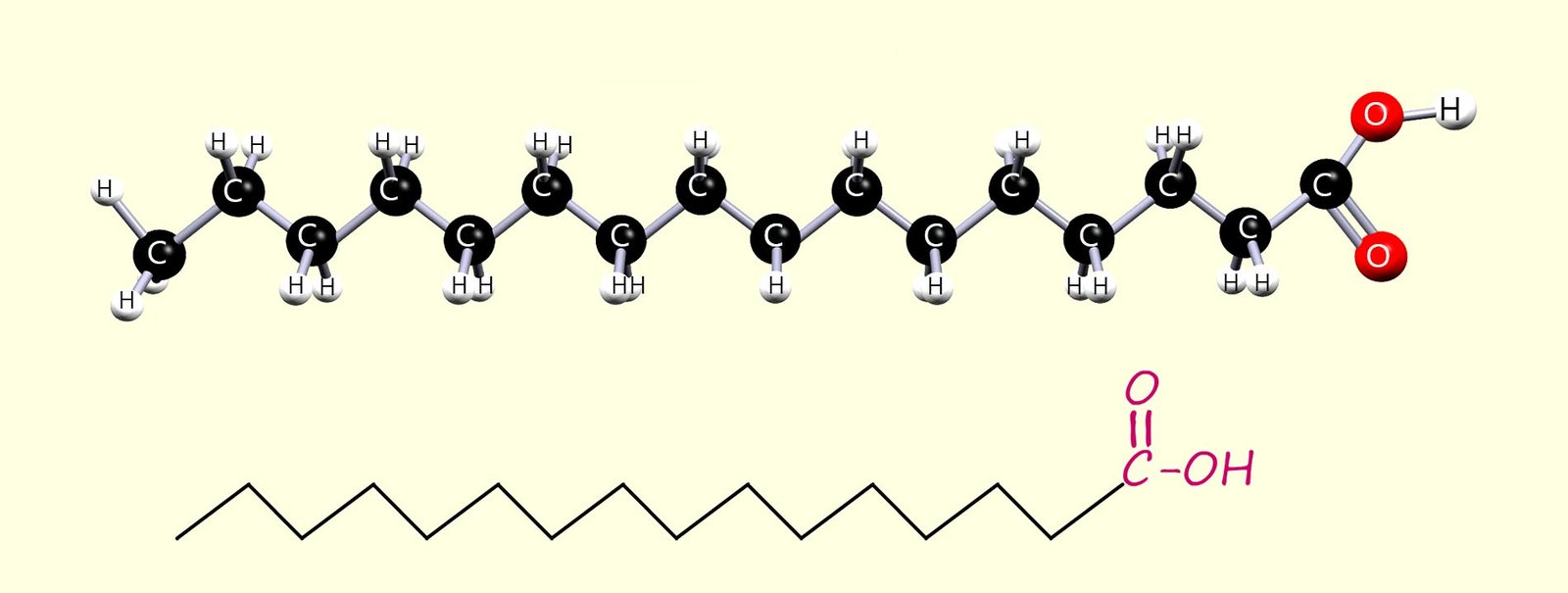
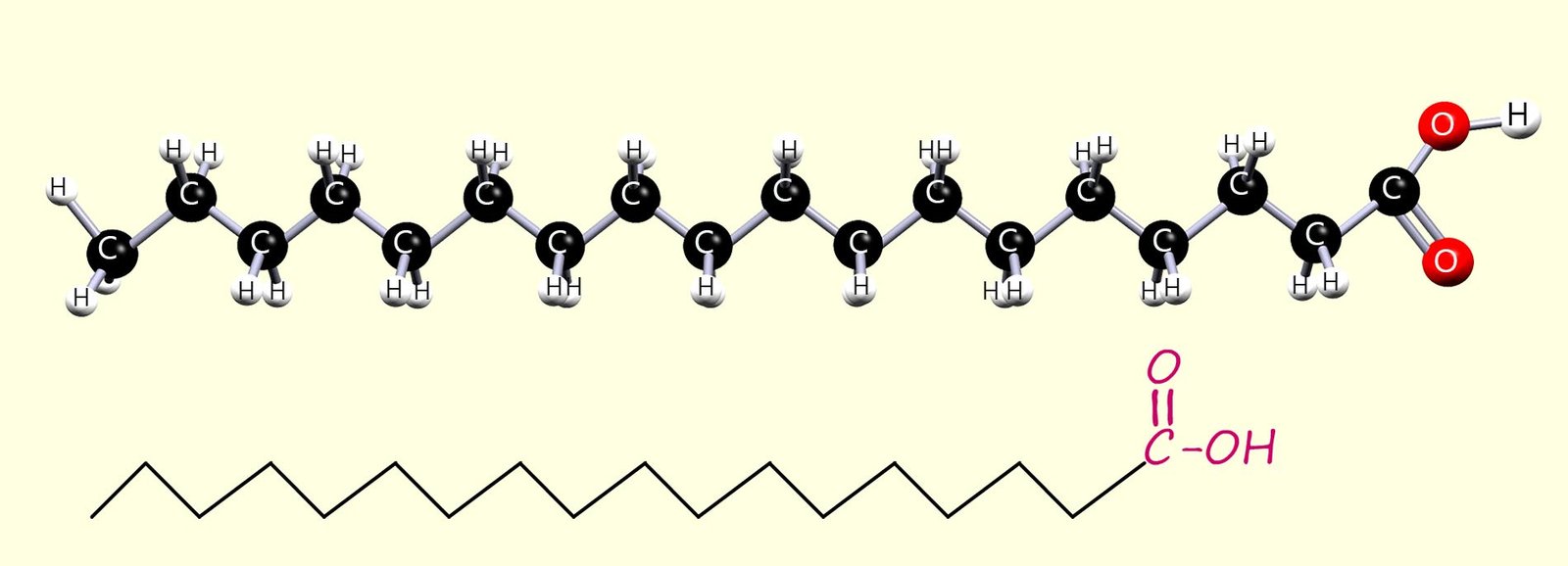
The Fatty acids found in oils and fats are long-chain carboxylic acids; usually with between 12 and 20 carbon atoms. Some of these fatty acids are saturated, some are unsaturated, and some are polyunsaturated (contain more than one C=C bond per molecule). Two common fatty acids found in fats and oils are:
Glycerol – A small alcohol molecule with three hydroxyl (–OH) groups. It reacts with fatty acids to form fats and oils.
Fatty acid – A long-chain carboxylic acid found in fats and oils. Fatty acids can be saturated, unsaturated or polyunsaturated.
Triester – An ester formed when one molecule of glycerol reacts with three fatty acids. Fats and oils are triesters.
Polyunsaturated – Describes a fatty acid that contains more than one carbon–carbon double bond (C=C) in its hydrocarbon chain.
Hexadecanoic and octadecanoic fatty acids are perhaps two of the best know fatty acids with many important uses that include:
In the two examples above both of the fatty acids were
saturated; that is they contain only carbon-carbon single bonds (C-C).
However fatty acids can also be
unsaturated and some fatty acids also have multiple C=C bonds; that is they are polyunsaturated.
Saturated long-chain fatty acid molecules as you can see in the images of hexadecanoic and octadecanoic acids have a fairly linear shape; that is they are reasonably straight molecules. This linear shape means that the molecules can pack closely together.
This close packing of the fatty acid molecules will increase the strength of the intermolecular forces between neighbouring molecules; this will result higher
melting points. For example stearic acid has a melting point of 70oC,
while the smaller palmitic acid has a melting point of 63oC; while an unsaturated fatty acid such as oleic acid (C18H34O2); which is found in olive oil has a melting point of about 140C. These melting points mean that the two fatty acids will be solids at room temperature while oleic acid will be a liquid.
As mentioned above, fats and oils are triesters formed when the alcohol or triol glycerol (propane-1,2,3-triol) reacts with long-chain carboxylic acids (fatty acids). This is a condensation or esterification reaction where each hydroxyl functional group (-OH) on the glycerol molecule reacts with the carboxyl group (-COOH) of a fatty acid, producing water and an ester linkage. The triester molecule formed is often called a triglyceride. The formation of a typical triglyceride molecule is outlined below:
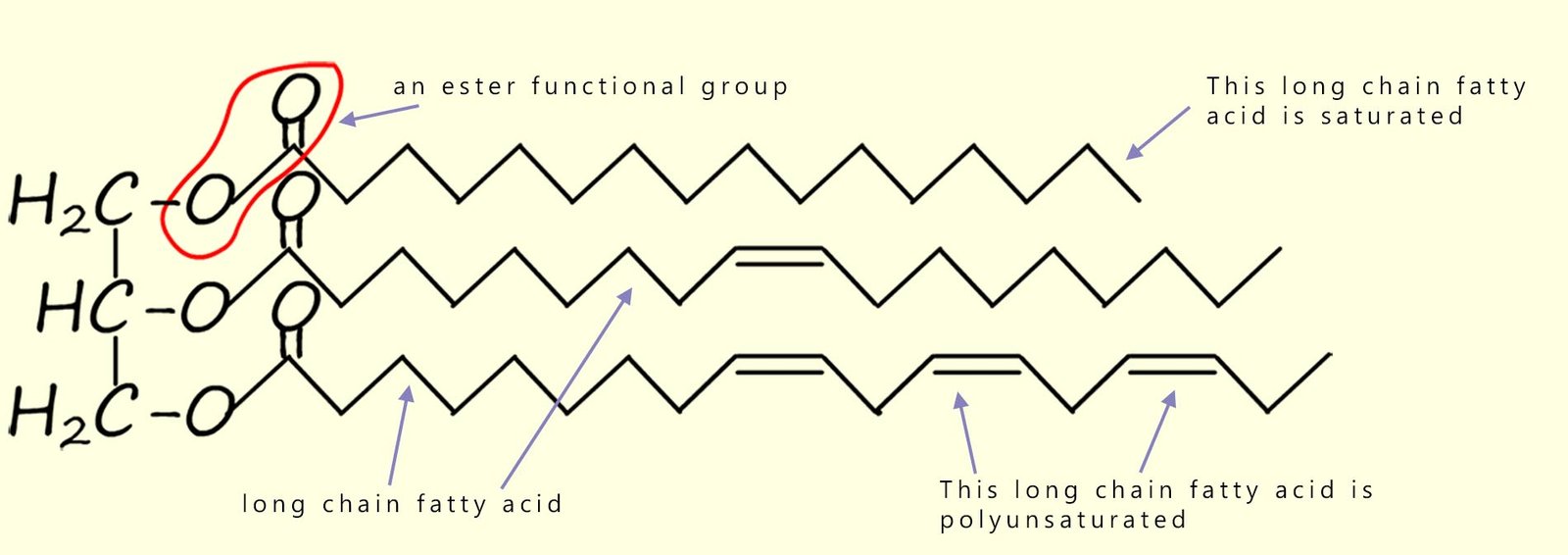
The structure of a typical vegetable oil molecule is shown in the image below. In this molecule you should be able to identify the three ester groups and the three long-chain carboxylic acids (the fatty acids) which make up this particular vegetable oil. In this particular oil one of the long-chain carboxylic acids is saturated, one has a single site of unsaturation and the bottom fatty acid hydrocarbon chain has multiple sites of unsaturation; that is it is polyunsaturated. In any one particular triglyceride molecule, the three fatty acids could be different or they could all be the same.
Fats and oils have similar structures but differ mainly in their melting points. Fats are solids at room temperature and tend to contain a higher proportion of saturated fatty acids, while oils are usually liquids at room temperature and tend to contain a higher proportion of unsaturated fatty acids. Fats are often obtained from animals, while oils are obtained mainly from plants.
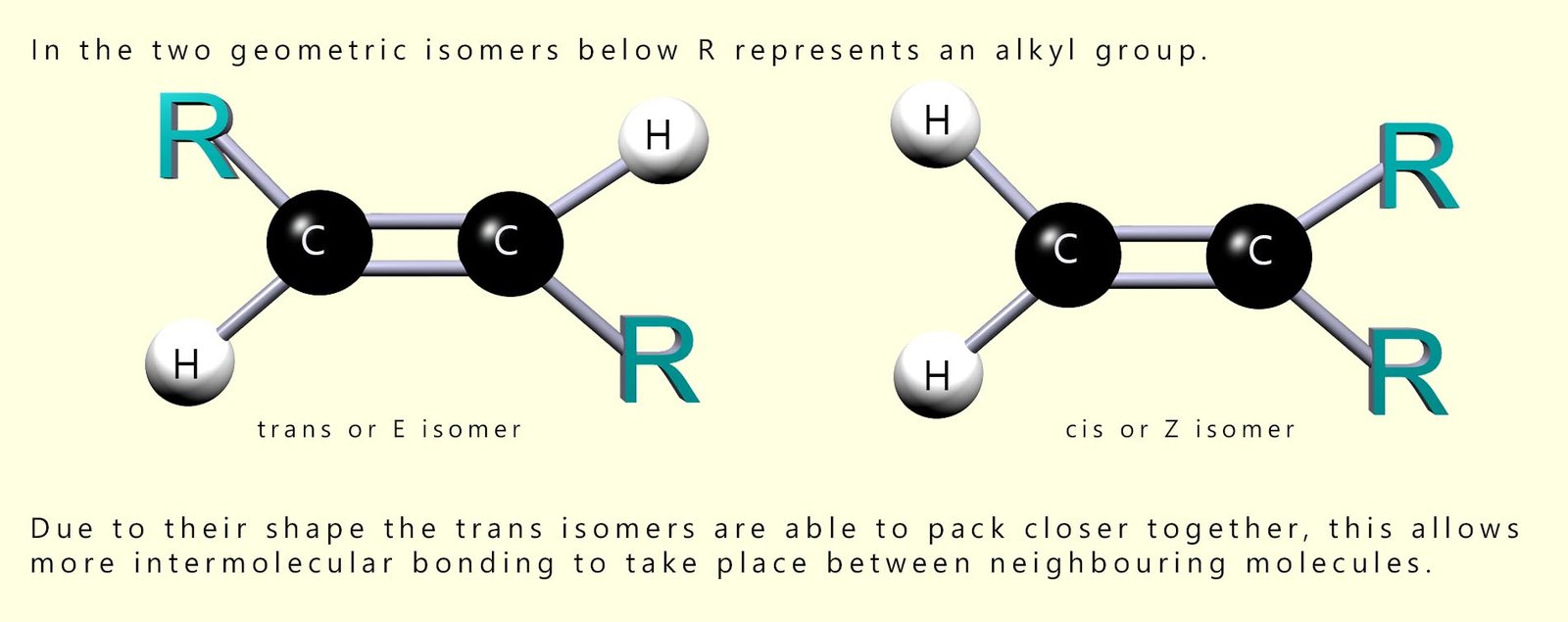
You may recall that there is the possibility of stereoisomerism with some unsaturated molecules; that is they may exist as a pair of geometric isomers (see the image below). Natural fatty acids in fats and oils mainly have the cis or Z arrangement around sites of unsaturation. One feature of the cis/Z shape is that the chains do not pack as closely as more linear trans/E geometric isomer. This means that the isomer with the cis/E arrangement have less intermolecular forces between adjacent molecules and therefore lower melting point.
Trans isomers are more linear or straight than cis isomers and so can pack more closely together, so they usually have stronger intermolecular forces and higher melting points. Oils are liquids and fats are solids at room temperature largely because oils contain a higher proportion of unsaturated fatty acids.
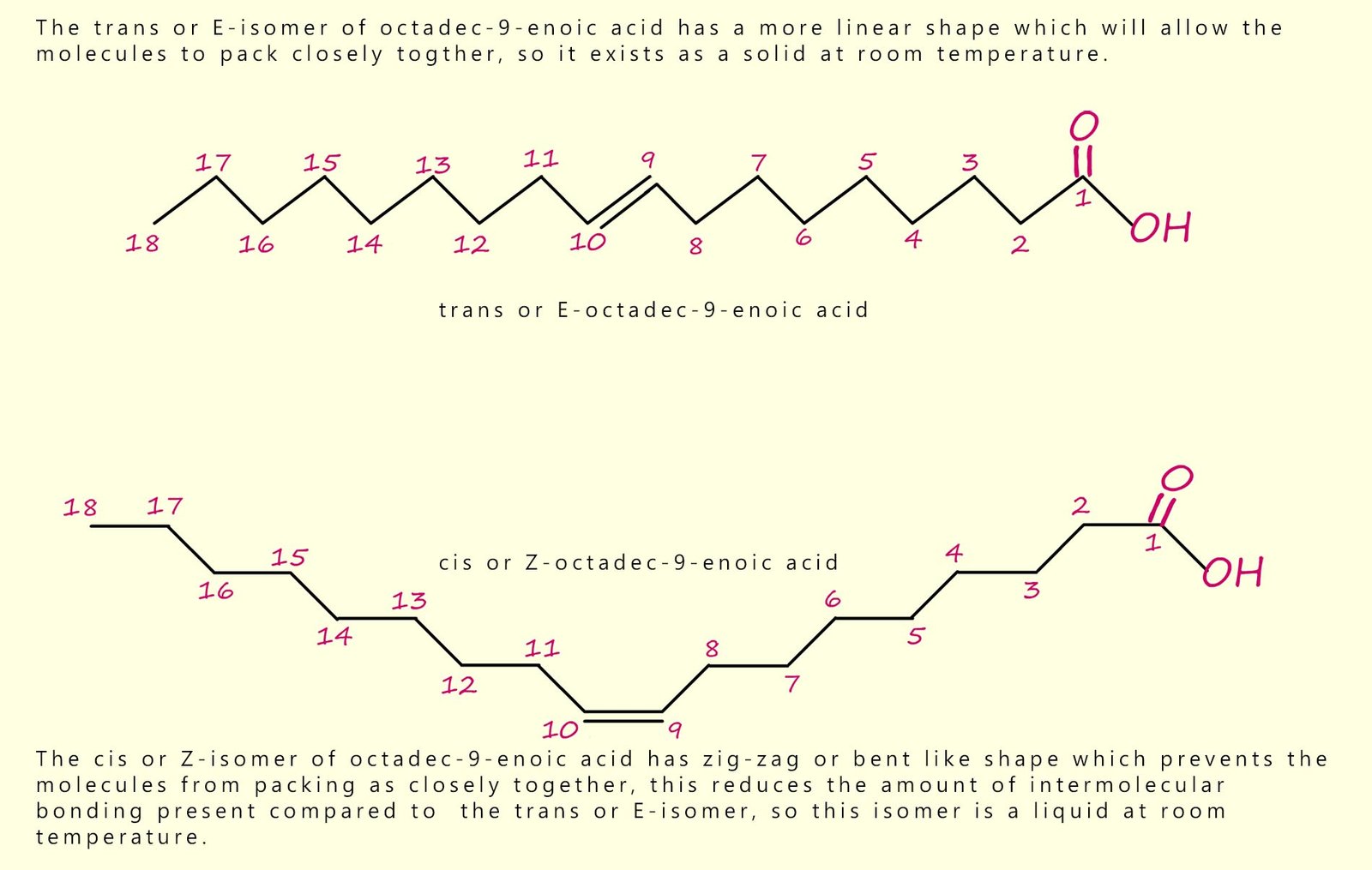
As an example, consider the two molecules shown in the image below. They are the cis and trans isomers of octadec-9-enoic acid (octadec = 18 carbons; en = C=C; 9 gives the position of the C=C). The intermolecular forces include hydrogen bonding between the carboxylic acid groups, and significant van der Waals forces between the long hydrocarbon chains. The difference in shape affects how closely the molecules pack, and therefore affects melting point.
Let's look again at an image of the structure of a typical vegetable oil we saw above, it is shown below again for reference. In this image you should be able to identify the three ester groups and the three long-chain fatty acids which make up this particular vegetable oil.

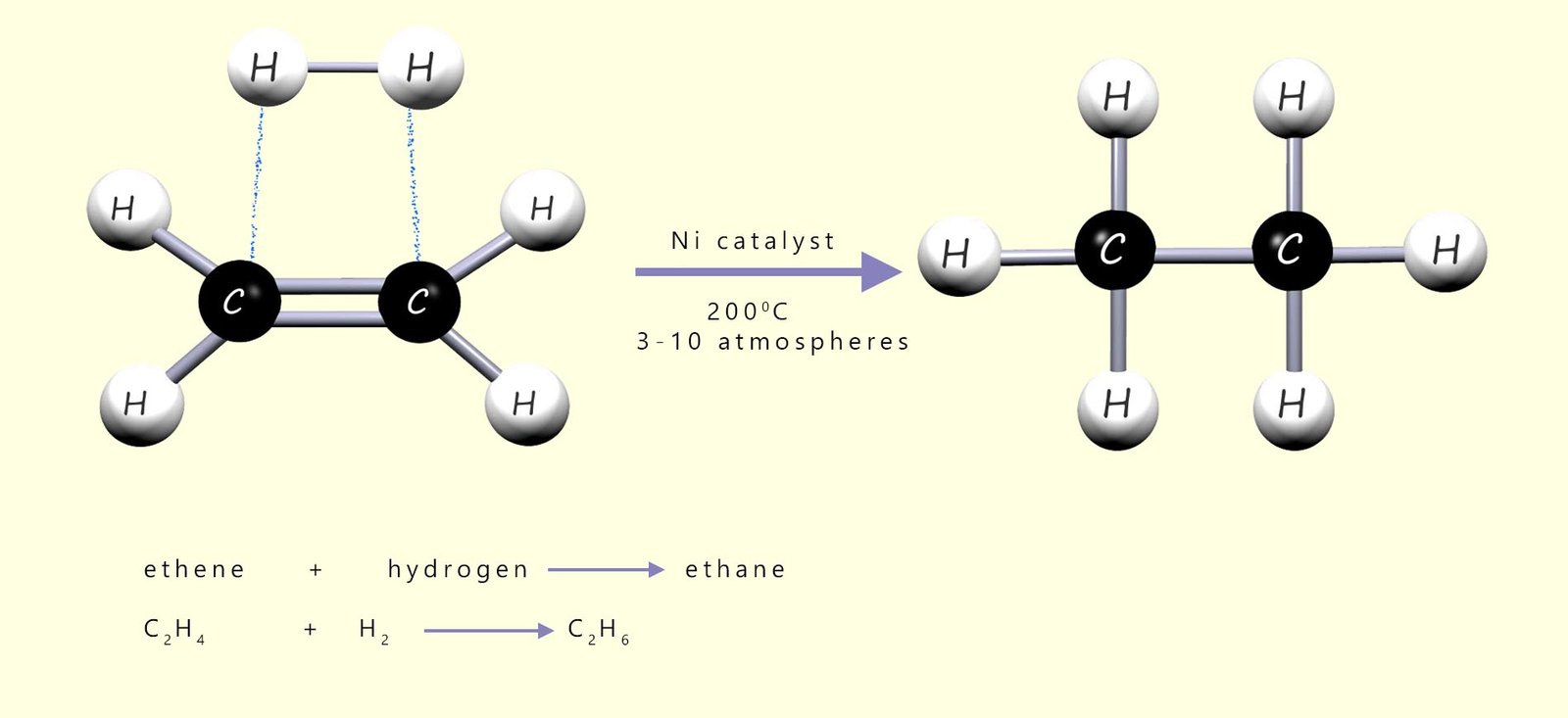
The sites of unsaturation present in oils and fats can be reduced by adding high-pressure hydrogen (H2) across the C=C bonds in the long unsaturated hydrocarbon chains; as a simple example consider the addition of hydrogen gas (H2) to the unsaturated molecule ethene to form ethane, as shown below:
If the addition of hydrogen gas is repeated using an oil or a fat, it will obviously reduce the amount of unsaturation present; this will likely increase the strength of the intermolecular forces present and therefore increase the melting point. Depending on how many C=C bonds are reduced to C-C bonds the product of this hydrogenation reaction can be a solid or a semi-solid fat such as margarine. This hydrogenation of vegetable oils is often called hardening and it typically requires a metal catalyst and a temperature of about 200°C and pressures in the range of about 3-10 atmospheres; although the exact conditions depend on the catalyst used and the amount of hydrogenation required; the pressure for example can be increased up to around 20 atmospheres if a large amount of hydrogenation is required.

One use of the hydrogenation reduction reaction is in the manufacture of margarine.
Margarine can be made from vegetable oils; for example, rapeseed or sunflower oil.
Now as we have seen above vegetable oils often contain a significant proportion of unsaturated long chain fatty acids.
The presence of these sites of unsaturation (C=C) in the vegetable oils lowers the melting point, which is why many
vegetable oils are liquids at room temperature.
However, by removing some or all of the sites of unsaturation by hydrogenation, the melting point can be raised.
If all the C=C bonds are hydrogenated, the product is likely to be a hard, saturated fat.
If only some C=C bonds are hydrogenated, it is possible to control how soft and spreadable the margarine will be.
Natural unsaturated fatty acids typically have a cis/Z configuration. When polyunsaturated oils are partially hydrogenated, some C=C bonds are end up switching configurations from the naturally occurring cis/Z configuration to the trans/E configuration. This isomerisation usually occurs during the hydrogenation process on the catalyst surface or through high-heat exposure during deep-frying or cooking. This isomerisation process occurs as follows:
During the hydrogenation process the vegetable oil is exposed to a hot nickel catalyst, this results in:


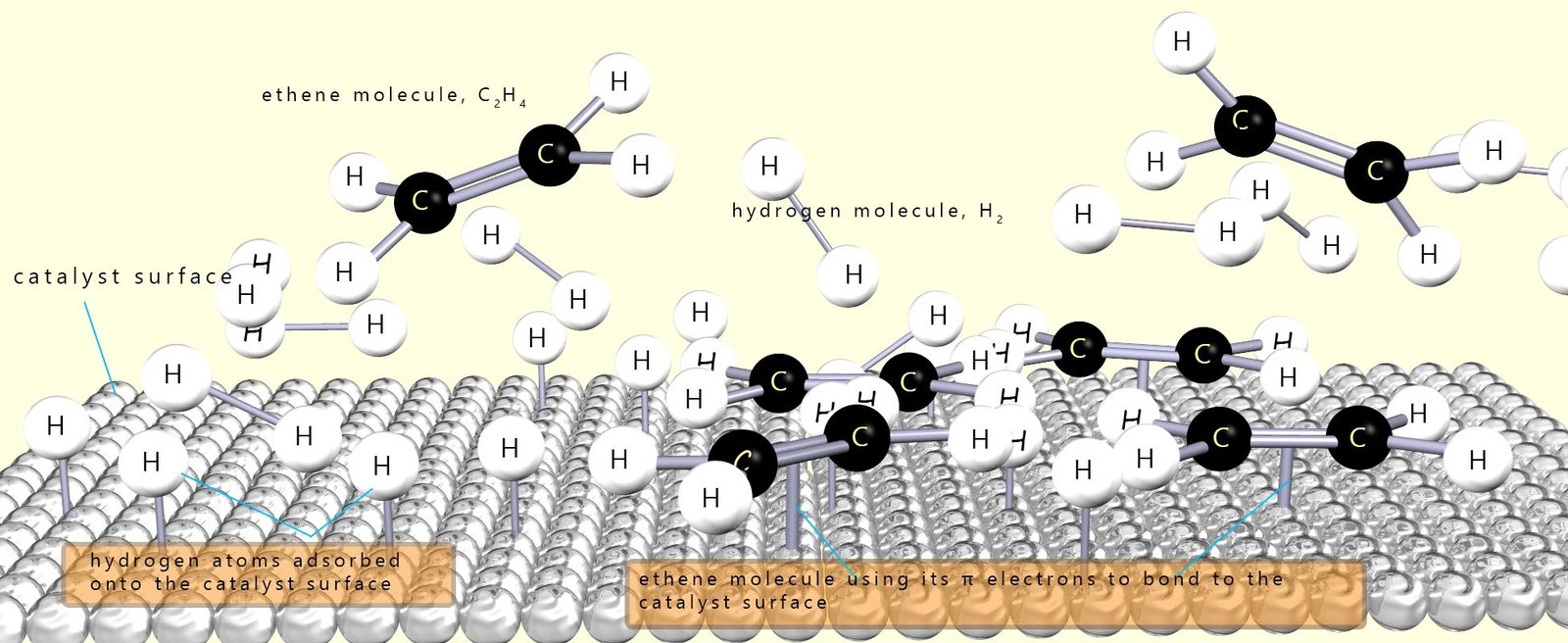
As a simple example of what happens on the catalyst surface, consider the hydrogenation of ethene gas (C2H4) to form ethane (C2H6), which is outlined in the image below. In the image you can see that the hydrogen molecules adsorb onto the catalyst surface and that the bond between the two hydrogen atoms in the molecule is broken and this results in the formation individual hydrogen atoms which are free to diffuse (move) across the catalyst surface.
The ethene molecules or it could be the oil molecules also bond to the catalyst surface using their pi electrons in the carbon carbon double bond (C=C). Once the alkene adsorbs onto the catalyst surface, the pi bond of the C=C is broken as its electrons interact with the metal surface. The sigma bond remains intact, so the carbon–carbon bond behaves like a single bond and free rotation can occur. Now once the reactants are adsorbed onto the catalyst surface there are two possible outcomes or pathways:
A crucial point here is that the alkene (or adsorbed oil molecule) doesn’t stay on the catalyst surface for long. If it desorbs before full hydrogenation can occur, it is more likely that the oil molecule will leave in the trans/E configuration that is as a trans fat.
The change from a kinked shape in the cis/Z isomer to a straighter, more linear shape in the trans/E isomer has a massive impact on the physical properties of the vegetable oil. The newly formed trans isomers can pack together much more closely and their density will therefore increase. This close packing increases the amount and strength of the Van der Waals (intermolecular) forces present between adjacent molecules. The most obvious changes are in the melting points of the oils, which are raised considerably; infact the liquid oils are likely to become solid fats- trans fats.
Complete the quick quiz below to review your understanding of trans fats
For each situation below, decide whether it is likely or unlikely to contain trans fats. Then press Check.
Lipoproteins are tiny particles in the blood that transport fats and cholesterol. This is necessary because fats and cholesterol are not soluble in water and cannot travel freely in the bloodstream.
A lipoprotein has a water-friendly outer layer made from phospholipids and proteins, which surrounds a fat-rich core containing triglycerides and cholesterol. This structure allows hydrophobic molecules to be carried safely in blood.
LDL mainly transports cholesterol from the liver to body tissues, while HDL removes excess cholesterol from the blood and carries it back to the liver. Problems arise when LDL levels are high and HDL levels are low.
Many cheap and highly processed foods may contain partially hydrogenated oils or trans fats. When you consume these isomerised fats and oils, your body tries to incorporate them into your cell membranes. In the body, lipoproteins are used to transport fats and cholesterol through the bloodstream, as these fats and cholesterol are not soluble in water. Cholesterol is an essential molecule within the human body, but problems arise when it is transported or stored in excess. The main roles of cholesterol within the body are shown in the enrichment activity below.
There are two main types of lipoprotein in the body involved in this process of transporting cholesterol, these are:
Cholesterol is an important and essential molecule in the human body, some of its main roles are listed in the table below:
| Function | Main roles of cholesterol |
|---|---|
| Cell membranes | Cholesterol is an essential part of animal cell membranes, it makes up about 30% of cell membranes where it helps control membrane structure and function. It helps keep membranes flexible in cold conditions and stabilises them at higher temperatures, helping to prevent membranes becoming too leaky. |
| Hormones | Without cholesterol the body could not produce many important chemical messengers that regulate growth, metabolism and reproduction. These include cortisol and the sex hormones oestrogen, progesterone and testosterone. |
| Digestion and fat absorption | The liver converts cholesterol into bile acids which are stored in the gallbladder and released into the small intestine. Bile acids help break large fat droplets into smaller ones so digestive enzymes can act more effectively. Without bile acids the body cannot efficiently absorb fat-soluble vitamins such as vitamins A, D, E and K. |
| Vitamin D synthesis | Cholesterol is the starting material for vitamin D synthesis. When the skin is exposed to ultraviolet radiation, a cholesterol-derived precursor is converted into vitamin D, which is essential for bone health and immune function. |
Foods that contain trans fats are sometimes called “Frankenstein foods” because they have been chemically altered from their natural form. In nature most unsaturated fats found in plants and animals have cis carbon–carbon double bonds, which give oils their natural liquid properties. During food processing vegetable oils such as sunflower and palm oil may be partially hydrogenated, converting some cis double bonds into trans double bonds that are rarely found in natural foods.
This small change in structure has a large effect on properties. Trans fats are straighter than cis fats, allowing their molecules to pack more closely together. As a result they behave more like saturated fats, with higher melting points and a more solid texture. This made them attractive to food manufacturers as they improve shelf life and texture and are cheaper than traditional animal fats.
The table below shows some of the main consequences of consuming processed foods that contain trans fats.
| Function | Main effects of trans fats |
|---|---|
| Double trouble |
Trans fats affect cholesterol levels in two harmful ways:
|
| Cardiovascular disease and stroke | Trans fats increase the risk of cardiovascular disease by promoting plaque build-up in arteries. This process, known as atherosclerosis, narrows blood vessels and restricts blood flow. As a result the risk of coronary heart disease and stroke is increased. |
| Type 2 diabetes | Trans fats can interfere with normal cell membrane structure, making membranes more rigid. This may contribute to insulin resistance, which is associated with an increased risk of developing Type 2 diabetes. |
As a consequence of these health risks many countries now restrict the use of industrial trans fats in food products, and dietary advice is to keep consumption of these “Frankenstein foods” as low as possible.


Fats and oils in terms of a healthy food type have a bit of a bad reputation; however they are
essential for normal healthy bodily functions. They provide energy, support cell growth,
protect organs, help with absorbing certain vitamins (A, D, E, K) and are important for producing hormones.
They also help maintain healthy skin and regulate body temperature. For example, a layer of fat offers
some protection to internal organs and fat acts as an insulator
to help maintain body temperature. Fats are also an important energy store.
Fats and oils are a valuable source of some essential
fatty acids which the human body cannot make for itself. They are also important to ensure that
vitamins A, D and E are effectively absorbed into the body. Vitamins A, D and E are fat-soluble and cannot be
properly absorbed unless a small amount of fat is present in the diet.
However too much fat in your diet; especially saturated fats
can raise cholesterol which increases the risk of heart disease.
A large percentage of the energy in our diet comes from fats and
oils. Sources of fats and oils include:
Unsaturated fats (including monounsaturated and polyunsaturated fats) are found mainly in plant foods and oily fish, for example:
Answer the questions below, they are designed to help you identify common mistakes and misunderstanding students often have:
Choose the best answer for each question. These are the mistakes that lose marks because they sound plausible.
The table below summaries the key points you should know for your exam.
| Key idea | What is happening chemically | Why it matters |
|---|---|---|
| Structure of fats and oils 🧬 | Fats and oils are triesters formed from glycerol and three long-chain fatty acids. These fatty acids can be saturated, unsaturated, or polyunsaturated depending on the presence of C=C bonds. | The presence or absence of C=C bonds controls molecular shape, packing, and physical properties such as melting point. |
| Saturated vs unsaturated fatty acids 🔗 | Saturated fatty acids contain only C–C bonds and are relatively linear. Unsaturated fatty acids contain one or more C=C bonds, usually in the cis/Z configuration, introducing bends in the chain. | Linear molecules pack more closely, leading to stronger intermolecular forces and higher melting points. |
| Cis and trans isomerism 🔄 | Cis/Z double bonds produce bent molecules, while trans/E double bonds produce straighter, more linear molecules. | Trans isomers pack more closely than cis isomers, increasing melting point and making oils behave more like saturated fats. |
| Catalytic hydrogenation ⚙️ | Hydrogen and the unsaturated molecule are adsorbed onto a metal catalyst surface. The C=C π bond is broken, allowing hydrogen to add across the double bond. | Hydrogenation reduces unsaturation, increasing melting point and turning liquid oils into semi-solid or solid fats. |
| Partial hydrogenation and trans fats ⚠️ | If an unsaturated molecule desorbs from the catalyst surface before full hydrogenation, the C=C bond can reform as trans/E rather than cis/Z. | This produces trans fats, which are rare in nature and linked to negative health effects. |
| Biological impact 🫀 | Trans fats alter membrane structure and affect how cholesterol is transported in the body via lipoproteins. | They raise LDL cholesterol and lower HDL cholesterol, increasing the risk of cardiovascular disease. |
As a final review answer the questions in the quick quiz below:
Answer these quick questions about why hydrogenation matters in real foods and in the body. Then click “Check answers” to see what you got right and wrong.
Why do food manufacturers like partially hydrogenated oils?
Why do trans fats often have higher melting points than cis fats with the same formula?
What is the key risk linked to consuming industrial trans fats?
During catalytic hydrogenation, what is true about the reactants on the metal surface?
What does “hardening” a vegetable oil usually mean?
Before you move on, can you tick all of these?
✅ If you can tick all of these, you’re in a strong exam position.