
A crystalline substance is one in which the atoms or ions that make up the structure are arranged in a very ordered and uniform way. A structure where the atoms or ions are arranged in a random or disordered way is called a non-crystalline or amorphous structure. As a rather simple example we could say a brick wall is crystalline, the brick are laid in a very ordered and regular way, if you knock the wall down then you end up with a large pile of brick, this would be a disordered or amorphous arrangement.
Most covalent substances have a molecular structure. This will consist of a small group of atoms with strong covalent intramolecular bonding and weak intermolecular bonding between different molecules. The forces between the molecules could be Van der Waals (London dispersion) forces or if the molecules have a permanent dipole then dipole-dipole interacts will be possible and for those molecules such as the alcohols, ammonia, hydrogen fluoride and water which contain a H atom bonded to either a N, O or F atom then hydrogen bonding will be possible. As a consequence of this small molecular size and weak intermolecular bonding most covalent molecular substances will be gases or volatile liquids with relatively low melting and boiling points.
Since molecular substance have only weak intermolecular bonding
it will not take much energy to
separate the molecules present by simply breaking these weak intermolecular bonds; so the melting and boiling points will be low when
compared to macromolecular structures with strong covalent bonds
in all directions and between all
the atoms involved in the structure.
Van der Waals forces exist between all atoms and molecules.
This form of intermolecular bonding is
very weak. It is caused by the formation of temporary induced dipoles within the atoms and molecules
as they
approach each other. The size of the attraction between the molecules and atoms increases with increasing
molecular size and as the number of electrons increases.
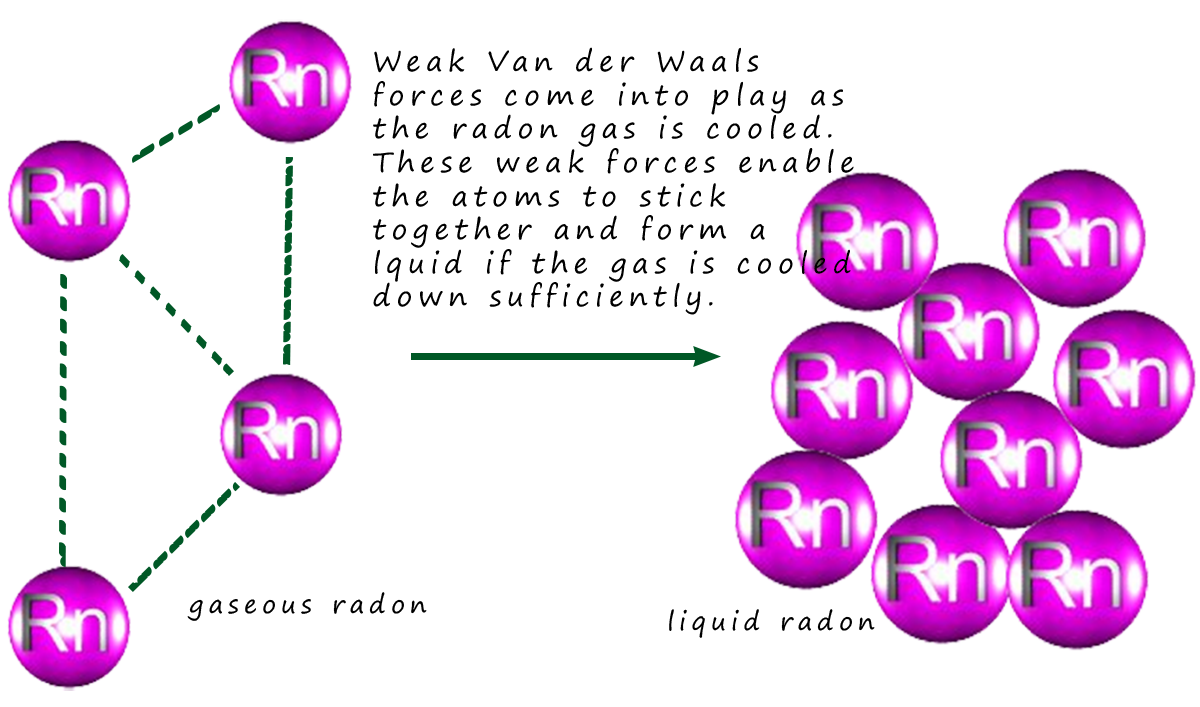
In the case of the noble gases the boiling and melting points
will increase from helium at the top of group 0 to
radon at the bottom. The reason for this is due to an increase in the amount and strength of the Van der
Waals bonding present as the atoms get larger (larger atoms means more electrons are present).
As another example consider the hydrogen halides, hydrogen fluoride, hydrogen chloride, hydrogen bromide
and hydrogen iodide.
| hydrogen halide | melting point/0C | boiling point/0C | dipole moment | ||
|---|---|---|---|---|---|
| H-F | -84 | 19 | large | ||
| H-Cl | -114 | -85 | ↓ | ||
| H-Br | -86 | -67 | ↓ | ||
| H-I | -56 | -35 | small | ||
Hydrogen fluoride despite being the smallest molecule has the highest boiling point. Remember that when a covalent substance changes state we DO NOT break the covalent bonds; it is the intermolecular bonds that break. In the case of hydrogen fluoride this will be hydrogen bonding, the strongest type of intermolecular bond. This hydrogen bond will outweigh any effects from the weak Van der Waals bonding present in this small molecule. As we descend the table above the size and mass of the molecules increase so the amount of Van der Waals bonding will increase; this will more than compensate for the reduction in the strength of the dipole-dipole bonding present.
Iodine consists of a small diatomic molecule held together by a strong covalent bond. Being a large atom with lots of electrons there is strong Van der Waals bonding between the iodine molecules, in fact the Van der Waals bonding is sufficiently strong that iodine is a solid at room temperature. The crystal structure of solid iodine is shown below:
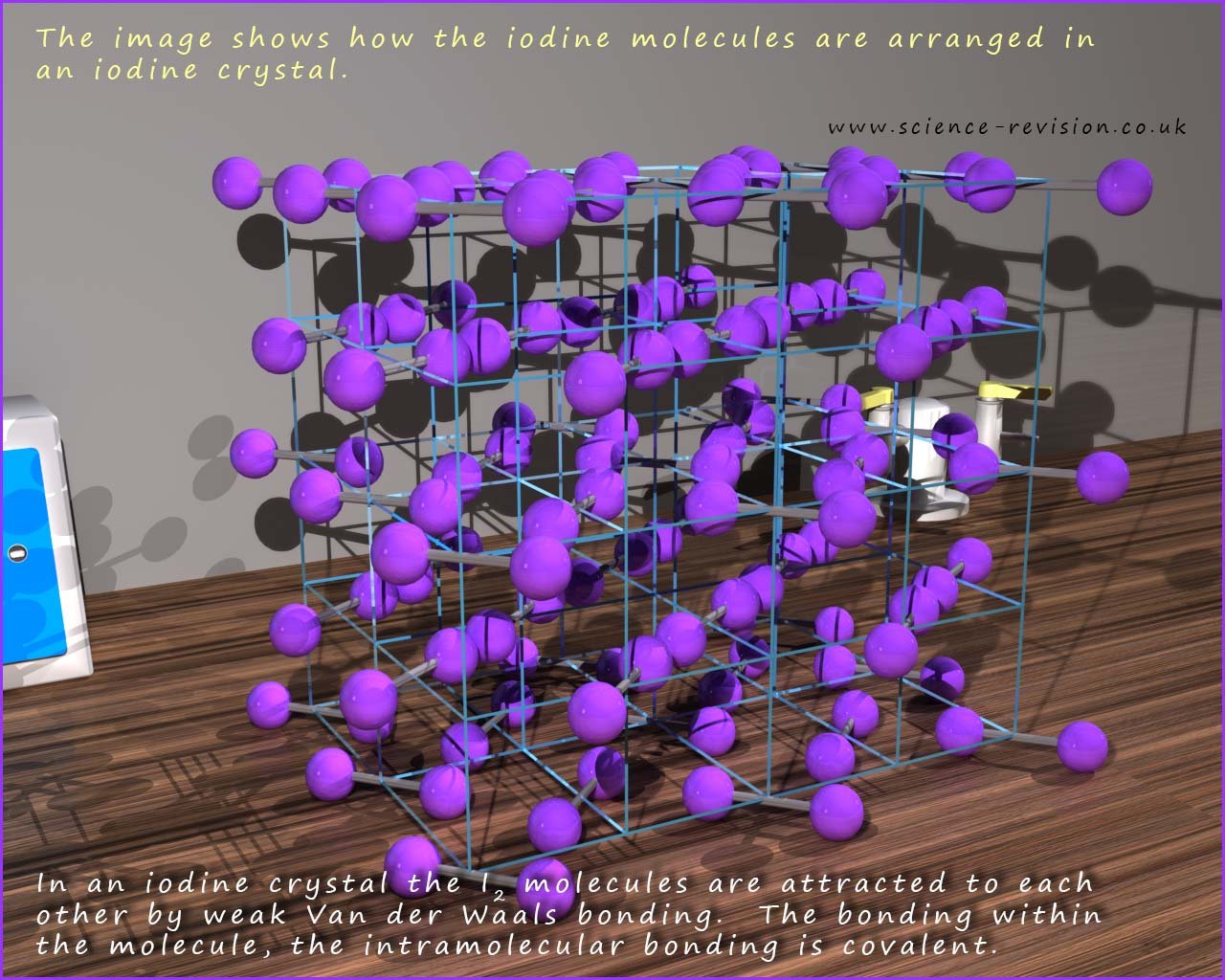
When solid iodine is heated it does not melt, in fact it changes directly from a solid to a gas; this state change is called sublimation. The reason for this unusual state change is that as the iodine crystal is heated and the iodine molecules start to vibrate more and more the weak intermolecular Van der Waals bonds are broken leaving the small individual diatomic iodine molecules which then enter the gas phase. The strong covalent bonds between the iodine atoms in the small iodine molecules are not broken when it is heated and sublimes.

 When considering if a solute will dissolve in a
solvent there are a number of
factors to consider. In order to
dissolve the solvent and solute must interact in some way;
this is most likely to be some sort of intermolecular
bonding. However you also need to consider the interactions between the solvent
molecules themselves and the
solute molecules.
When considering if a solute will dissolve in a
solvent there are a number of
factors to consider. In order to
dissolve the solvent and solute must interact in some way;
this is most likely to be some sort of intermolecular
bonding. However you also need to consider the interactions between the solvent
molecules themselves and the
solute molecules.
In order for a solute to dissolve the solvent
molecules will have to separate the solute particles from each other, that
is the solvent needs
to be able to overcome the intermolecular bonding holding the
solute particles together.
Similarly solvent-solvent intermolecular bonding
will need to be overcome before the solvent molecules can interact with
the solute.
Finally the solvent and solute molecules
need to be able to interact with each other. The type of interaction will
depend largely on the bonding present in the solvent and solute
molecules.
The image below shows some of the possible interacts between the solvent and
solute molecules:
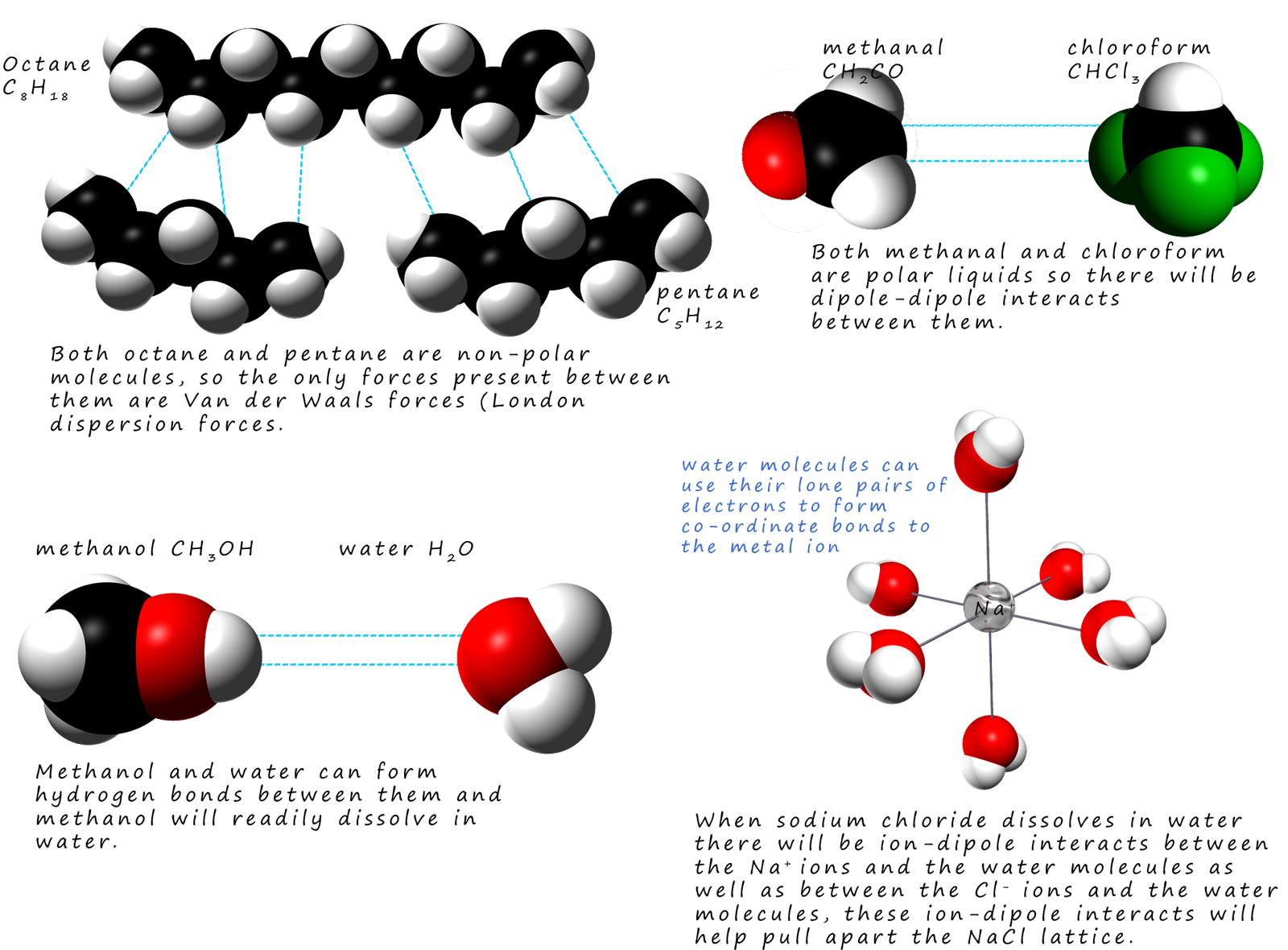
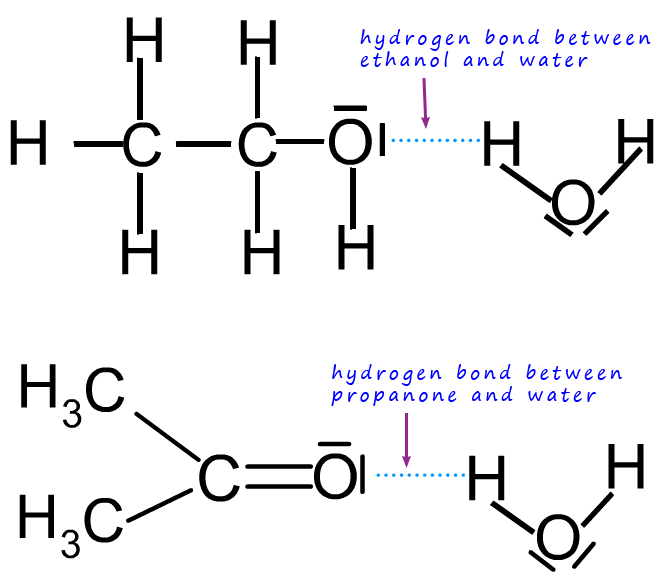 Perhaps the most commonly used solvent is water.
If a substance can form hydrogen bonds with water
then it is likely it will dissolve. In the example above ethanol and
water molecules both form hydrogen bonds between
themselves so when they are mixed to form a solution ethanol and water
will readily form hydrogen bonding
between them. Alcohols and short chain carboxylic acids such as ethanol and ethanoic are both soluble in
water since
the O-H group in both these molecules can form a hydrogen bond to a water molecule.
Perhaps the most commonly used solvent is water.
If a substance can form hydrogen bonds with water
then it is likely it will dissolve. In the example above ethanol and
water molecules both form hydrogen bonds between
themselves so when they are mixed to form a solution ethanol and water
will readily form hydrogen bonding
between them. Alcohols and short chain carboxylic acids such as ethanol and ethanoic are both soluble in
water since
the O-H group in both these molecules can form a hydrogen bond to a water molecule.