
Most covalent compounds involve bonding between atoms with different electronegativity values. This means that the electrons in any bonds formed will NOT be shared EQUALLY. In an ionic compound the ions formed have positive and negative charges because there is complete transfer of an electron from the metal to the non-metal atom. In a polar covalent bond or simply a polar bond the electrons are shared unequally between the two atoms or we could simply say one atom in the bond has a much larger share of the electrons. This means that the atoms involved in the polar covalent bond will have partial charges and not full positive and negative charges. The Greek symbol delta (δ) is used to show the resultant partial positive (δ+) and negative (δ-) charges that result from the unequal sharing of the electrons in a polar covalent bond. As an example consider the hydrogen chloride molecule (HCl) shown below:
 |
The large difference in the electronegativity values between the hydrogen and chlorine atoms in a molecule of hydrogen chloride means that the bond between these two atoms is a polar covalent bond. The 2 electrons in this bond will spend more time closer to the more electronegative chlorine atom giving it a partial negative charge (δ-), while the hydrogen atom will be left with a partial positive charge (δ+). |

|
While the small difference in electronegativity between a carbon atom and a hydrogen atom means that the bond between these 2 atoms is a covalent bond in which the 2 electrons in the bond are equally shared between the carbon and hydrogen atoms. |
Another way of thinking about polar covalent bonds is that the uneven distribution of electrons in a polar bond creates two poles, one slightly positive (δ+) and one slightly negative (δ-). These polar covalent bonds are said to have a permanent dipole, this simply means they have charged regions, one region with a partial positive (δ+) charge and another region with a partial negative charge (δ-) within the molecule.
The image below shows three polar molecules, that is molecules which have a slightly positive end and a slightly negative end due to uneven sharing of electrons within its chemical bonds. Hopefully you will see that the (δ-) end of the molecule is towards the more electronegative atom involved in the polar bond while the (δ+) end of the molecule is towards the less electronegative atom in the polar covalent bond.
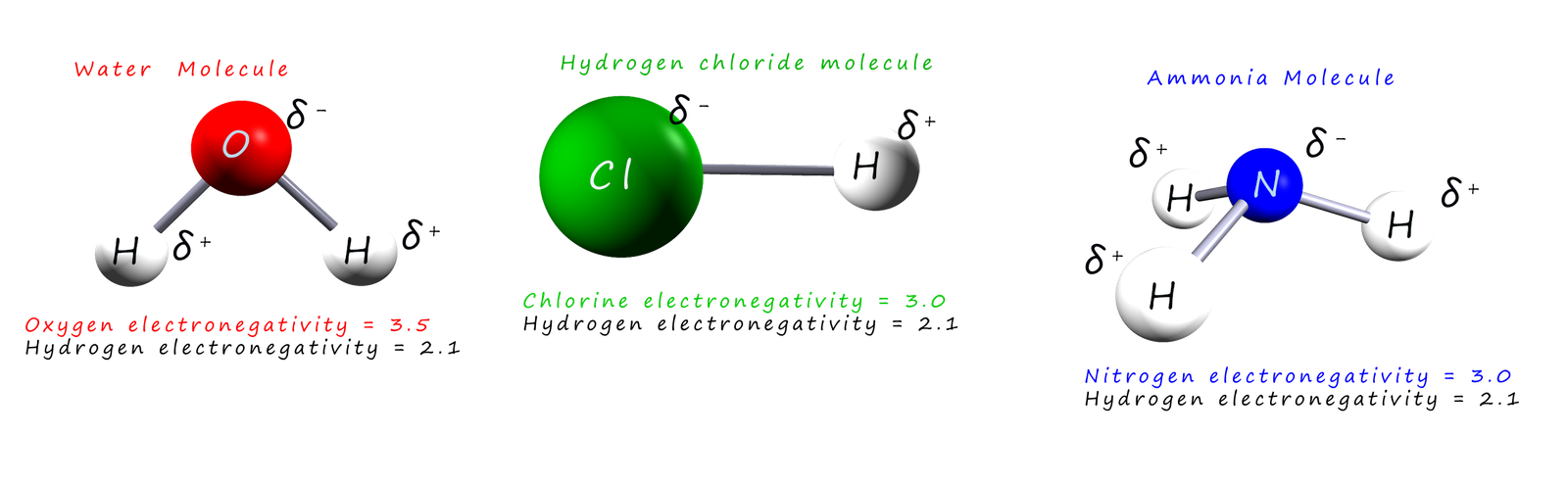
A polar covalent bond or simply a polar bond can be thought as a sort of halfway house between covalent and ionic bonding in that the electrons in the bond are shared but not equally as is the case in a covalent bond and the polar covalent bond contains atoms with partial charges, similar to those found in an ionic compound. However the charges are only partial charges and not full charges as is the case with ionic compounds. The charges on the atoms are only partial charges simply because the electrons are not completely transferred, just shared unequally.
The larger the difference in the electronegativity between the two atoms in the covalent bond the more polar the bond will be. If the bond between two different atoms has a very large difference in electronegativity values of say 1.7-2.0 or more it will be an ionic bond. If the differences in the electronegativity values are around 0.5 or less then the bond is likely to be covalent. Click on the link for bond character above or here for more information.
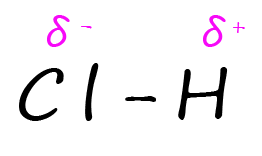 Molecules with permanent dipoles, that is a molecule which has an uneven distribution of electrical charge within it. One side has a slightly positive charge (δ+), and the other a slightly negative charge (δ-). Permanent dipoles occur where there are differences in the electronegative values of between
0.5 and 1.9 between the bonding atoms. This difference in electronegativity leads to formation of polar bonds and the molecule will have
partially charged ends (a dipole).
In the example above hydrogen chloride gas consists of small covalent molecules. Hydrogen has an electronegativity
value of
2.1 and chlorine has a value of 3.0; this means that the difference in electronegativity
is large enough to produce
a permanent dipole in the hydrogen chloride molecule. The electrons
in the H-Cl bond will spend most of their time
closer to the chlorine atom; this means it will have a partial (δ-) negative charge and the hydrogen atom will have
a partial positive charge (δ+).
Molecules with permanent dipoles, that is a molecule which has an uneven distribution of electrical charge within it. One side has a slightly positive charge (δ+), and the other a slightly negative charge (δ-). Permanent dipoles occur where there are differences in the electronegative values of between
0.5 and 1.9 between the bonding atoms. This difference in electronegativity leads to formation of polar bonds and the molecule will have
partially charged ends (a dipole).
In the example above hydrogen chloride gas consists of small covalent molecules. Hydrogen has an electronegativity
value of
2.1 and chlorine has a value of 3.0; this means that the difference in electronegativity
is large enough to produce
a permanent dipole in the hydrogen chloride molecule. The electrons
in the H-Cl bond will spend most of their time
closer to the chlorine atom; this means it will have a partial (δ-) negative charge and the hydrogen atom will have
a partial positive charge (δ+).
This means that when different hydrogen chloride molecules approach each other there will be an electrostatic attraction between the partially charged δ+ and δ- ends or poles of the molecules. This type of intermolecular bonding between these hydrogen chloride molecules is called dipole-dipole bonding. This is shown in the diagram below:
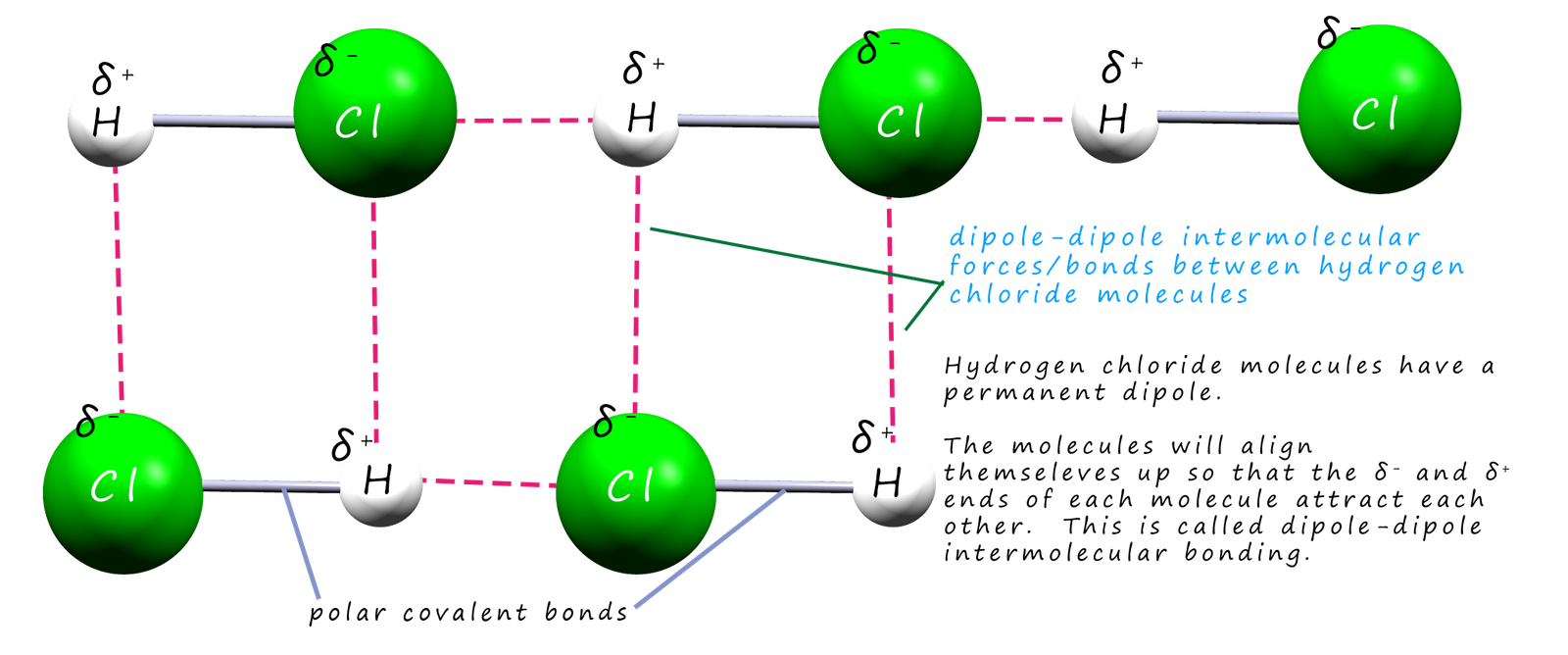
As mentioned above a permanent dipole occurs when the two atoms in a covalent bond have large differences in their electronegativity values, that is one atom in the bond has a slightly positive charge (δ+) and the other atom a slightly negative charge (δ_). Now molecules which contain a permanent dipole like hydrogen chloride above can interact with each other through dipole-dipole intermolecular bonds. However not all molecules which contain bonds with dipoles can interact with each other through dipole-dipole intermolecular bonds. Not only do we have to consider the polarity of the bonds in a covalent molecule but you must also take into account the shape of the molecule.
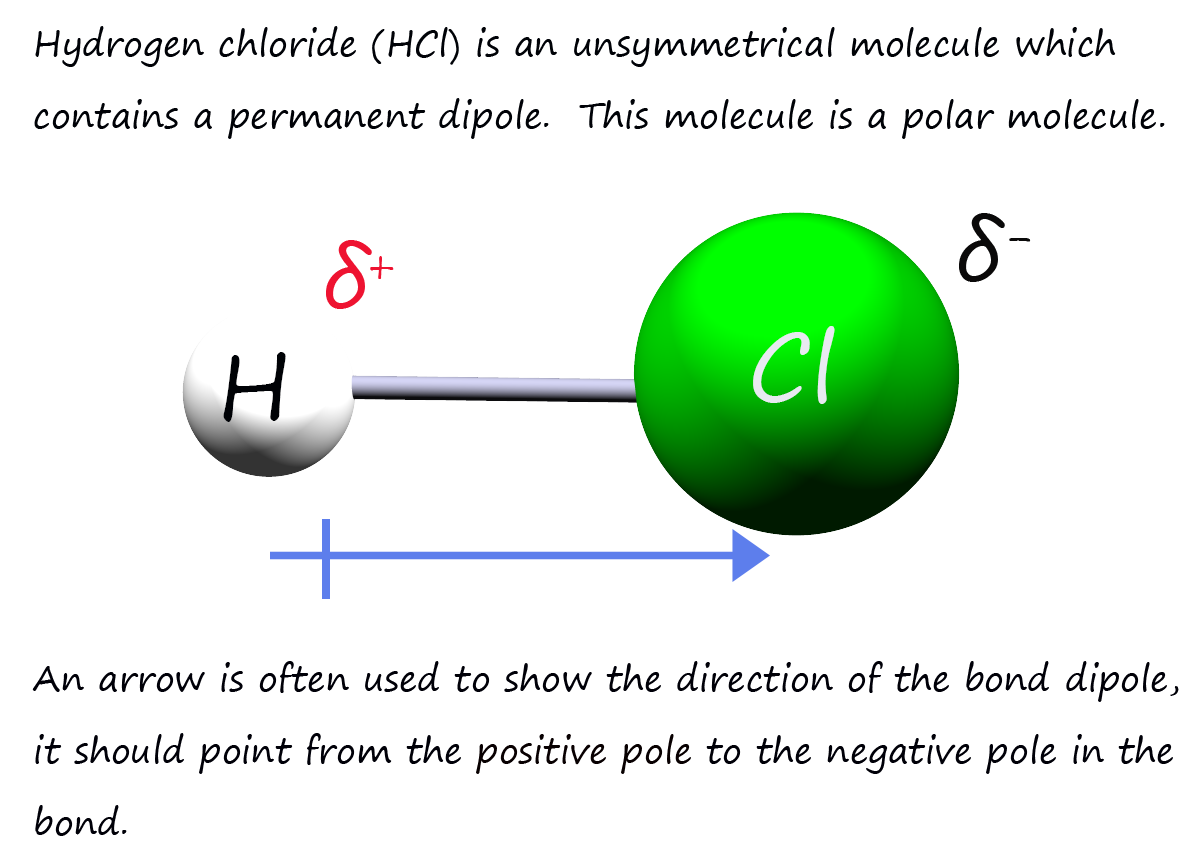
Now H-Cl is a non-symmetrical molecule with polar covalent bonds. We could also say that this is a polar molecule (that is a molecule with an uneven distribution of electrical charge or simply a molecule where one region has a partial positive charge (δ+ ) and the other a partial negative charge (δ-), this is shown in the image opposite.
Are all molecules which have polar covalent bonds polar molecules? The answer to this question is NO. To decide if a molecule is polar you not only have to decide if it contains polar bonds but you MUST also consider the shape of the molecule. Bond dipoles are vector quantities and have both a size and a direction. If the molecule has polar bonds but is highly symmetrical the chances are that the molecule will be non-polar even though it contains polar covalent bonds. As an example consider a molecule of carbon tetrachloride (CCl4) which is shown below:
 |
The carbon chlorine bonds are polar, the electronegativity of Cl is 3.0 and carbon is 2.5, this means that the chlorine atoms will be electron rich with a partial negative charge (δ-) and the carbon atom will be electron deficient with a partial positive charge (δ+).
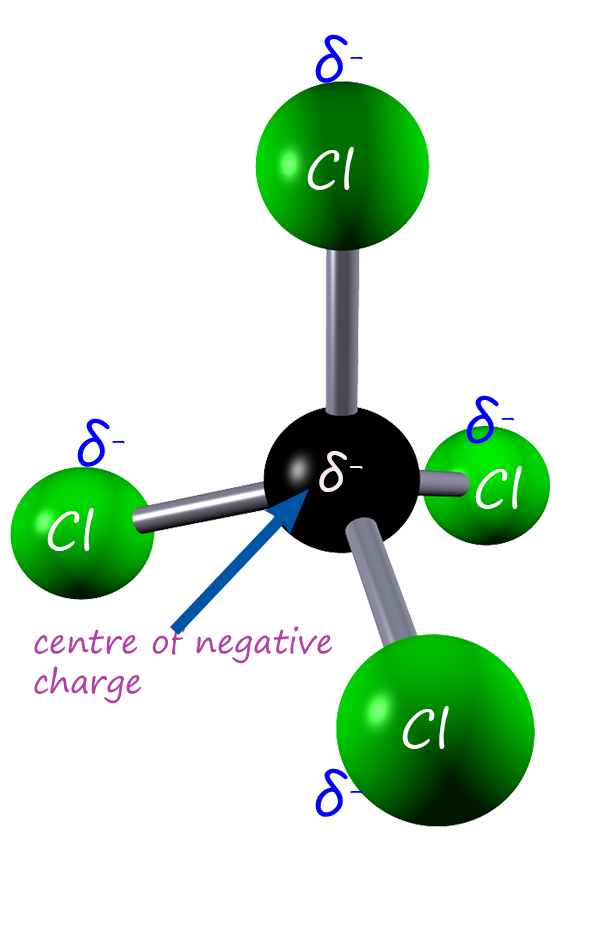
Each of the C-Cl bond dipoles are shown in the molecule on the left. Each of the chlorine atoms has a partial negative charge (δ-). You can see that carbon tetrachloride is a tetrahedral shaped molecule and as such is very symmetrical. So where would the centre of negative charge for this molecule lie? Well the centre of negative charge is at the central location of all the partial negative charges, that is directly over the carbon atom as shown in the image on the left. The centre of positive charge for this molecule is obviously over the central carbon atom. So for this molecule the centre of positive and negative charge lie directly over each other. This means that this molecule is non-polar, despite the fact it has polar covalent bonds. This means that carbon tetrachloride cannot undergo dipole-dipole intermolecular bonding. |
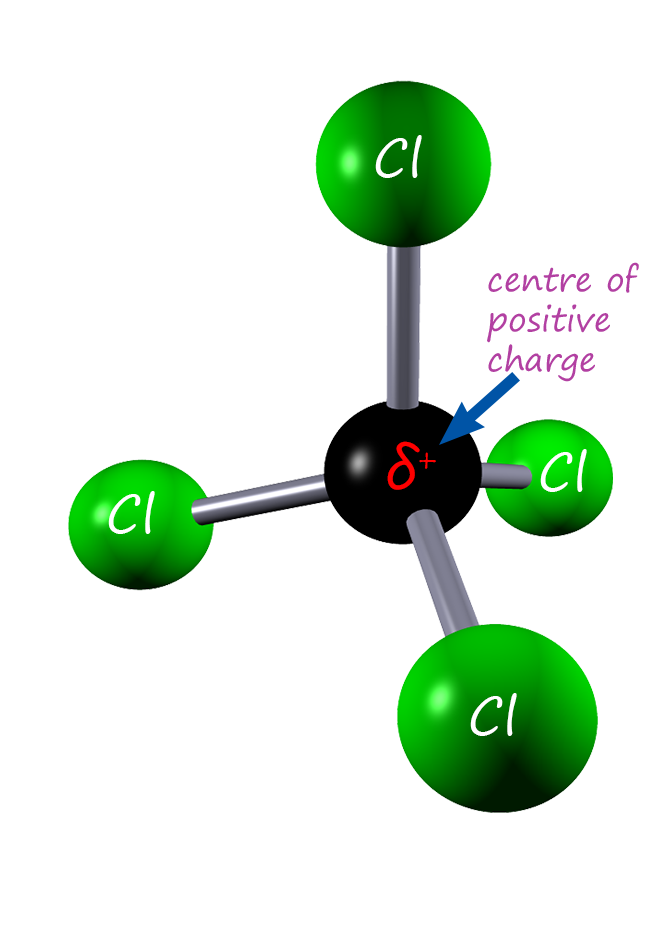 |
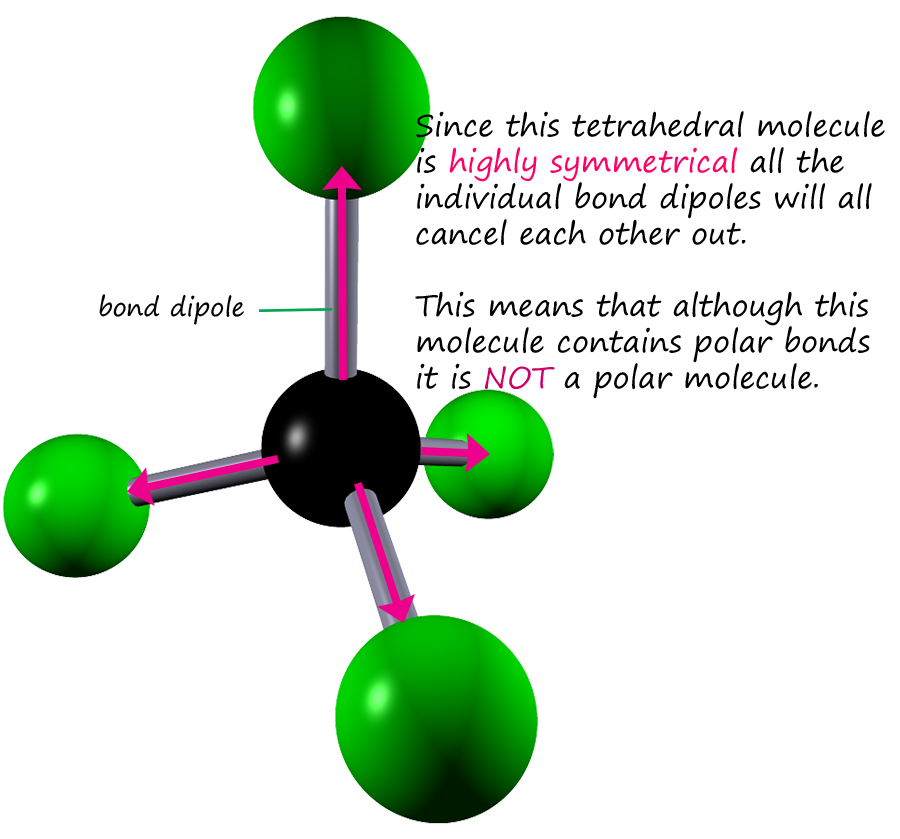
You may also see the carbon tetrachloride molecule drawn out using arrows to represent the bond dipoles (see the image opposite). This is often helpful
in deciding if the molecule overall will be a polar one with a dipole moment. The arrows representing the bond dipoles give
an indication of the size of the dipole present in any particular bond. I hope you can see that all the bond dipole arrows will effectively cancel each other out
due to the shape of this symmetrical molecule.
The carbon tetrachloride molecule was non-polar because the centre of positive charge on the carbon atom was directly on top of the centre of negative charge. The centre of negative charge was on top of the carbon atom due to the shape of the molecule. Now the carbon tetrachloride molecule has a tetrahedral shape and if you imagine the four chlorine atoms at the four corners of a tetrahedron then the centre of this tetrahedron will be on top of the carbon atom, that is the centre of negative charge lies directly on top of the centre of positive charge. Since the centres of positive and negative charge lie directly on top of each other carbon tetrachloride, despite having polar bonds is NOT a polar molecule.
The molecule has no overall dipole.
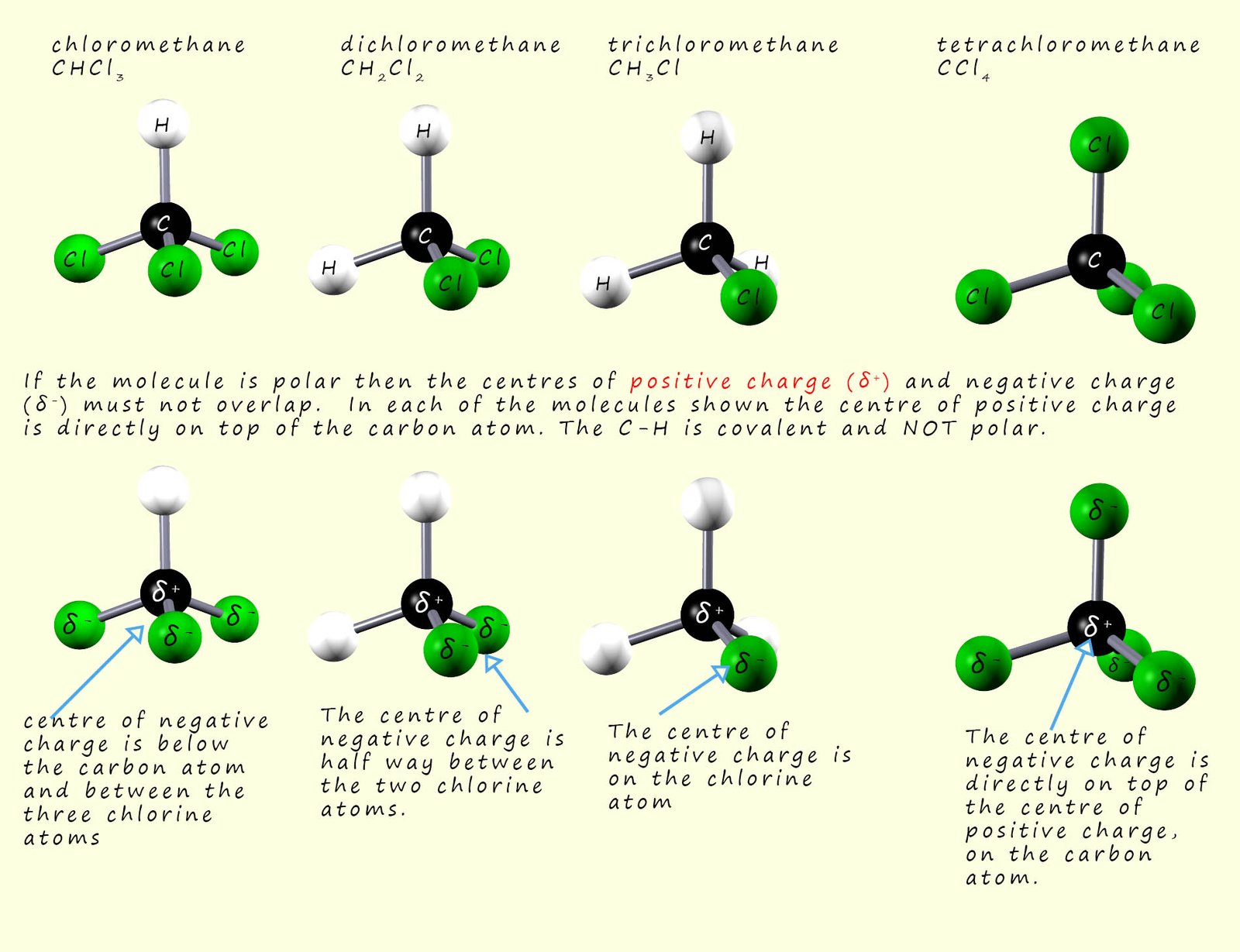
However consider the molecules shown below, are any of these molecules polar? Well all the molecules have an identical tetrahedral shape, they are all very symmetrical. The C-H bond is a covalent bond where the electrons in the bond are equally shared between the carbon and hydrogen atoms. However the C-Cl bond is polar as discussed above. Now if a molecule is polar then the centres of positive charge and negative charge must NOT overlap. So to decide if the molecules below are polar simply find the centres of positive and negative charge. This is usually fairly easy to do. The centre of positive charge for all the molecules is the carbon atom, which will have a
partial positive charge (δ+). The centre of negative for each of the molecules is also shown in the image below. So we can see that all the molecules except carbon tetrachloride are polar molecules since for all except carbon tetrachloride the centres of positive and negative charge do not coincide.
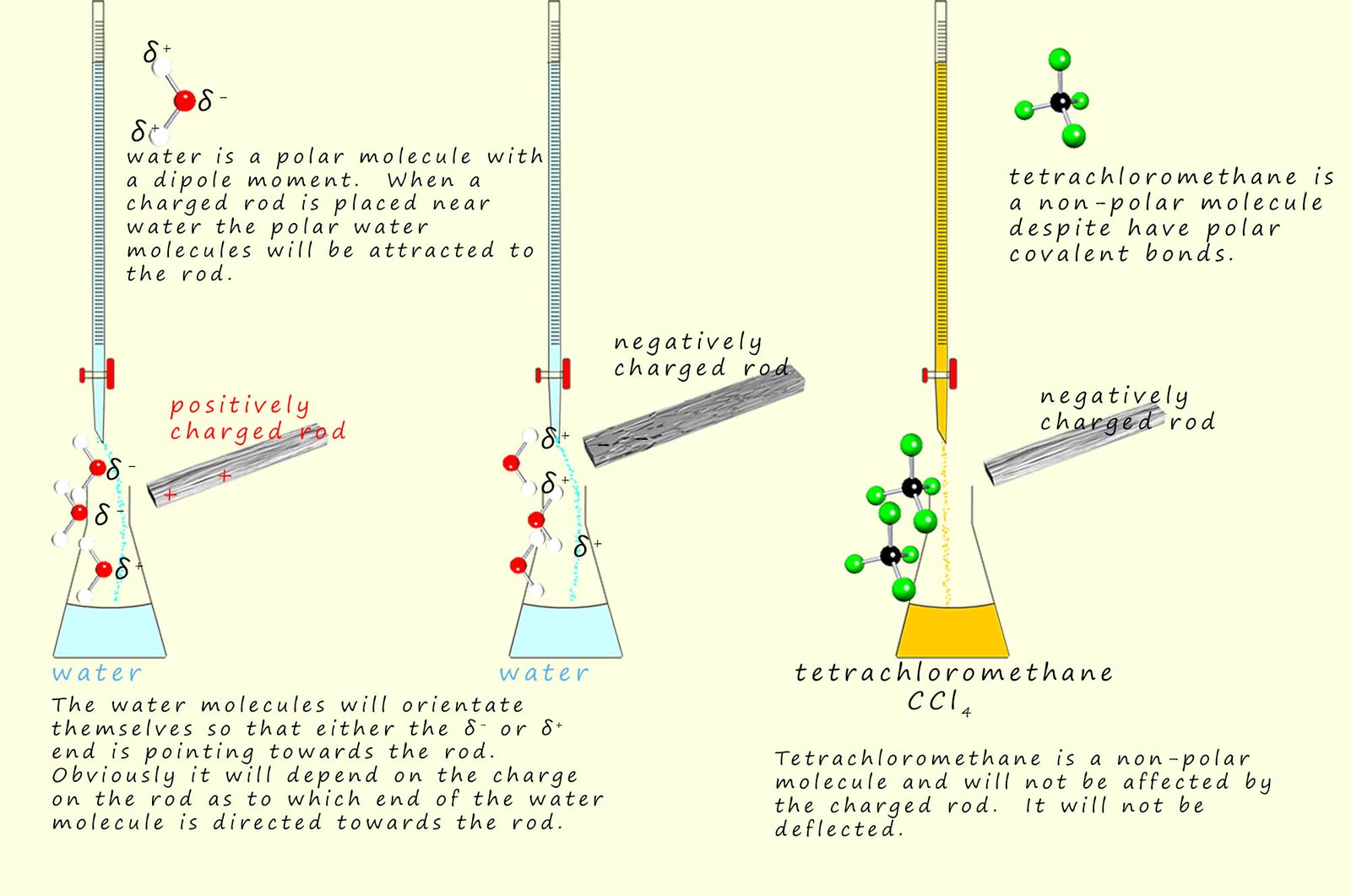
A fun way to find out if liquids are polar or not is simply to place them in a burette and have a slow steady flow of the liquid under test to leave the burette. If a charged rod is now brought up to the liquid flow then if the liquid is polar it will be deflected by the charged rod. However if the liquid is non-polar then it will be unaffected by the charged rod. This is outlined below: