

The period 3 elements (Na to Ar) have widely varying melting points; now the melting point of any particular element will largely be determined by the type of structure the element has; that is does it have a giant or molecular structure or even one consisting of monatomic atoms. The other factor to consider is the type of bonding present; both intramolecular and intermolecular bonding.
The period 3 elements have a wide variety of structures:
The table below shows the structure of all the period 3 elements from sodium (Na) to argon (Ar) along their melting points.
| Element | Symbol | Melting point (°C) | Structure / bonding | Structure |
|---|---|---|---|---|
| Sodium | Na | 98 | Giant metallic lattice structure (1 valence electron) and the sodium ions have a +1 charge |  |
| Magnesium | Mg | 650 | Giant metallic lattice structure (2 valence electrons) and the magnesium ions have a 2+ charge. |  |
| Aluminium | Al | 660 | Giant metallic lattice structure (3 valence electrons) and the aluminium ions have a 3+ charge. |  |
| Silicon | Si | 1410 | Giant covalent network structure, the structure of silicon is very similar to that of diamond. | |
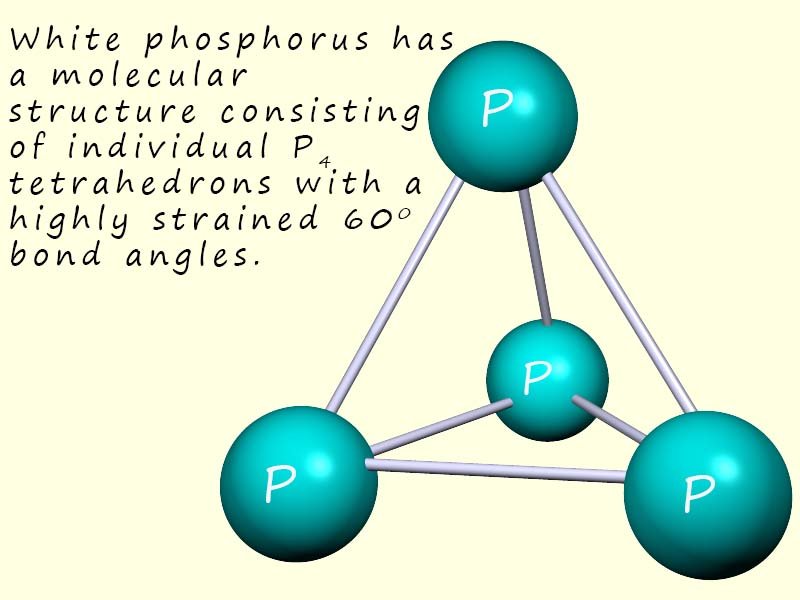
| Phosphorus (P4) | P | 44 | Simple molecular, white phosphorus consists of 4 phosphorus atoms in a tetrahedral arrangement. | 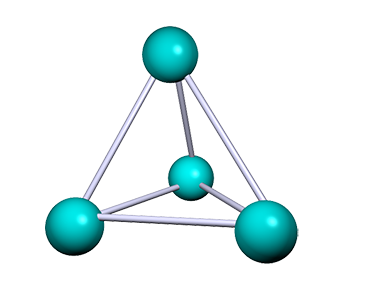 |
| Sulfur (S8) | S | 115 | There are many allotropes of the element sulfur- a common allotrope called rhombic sulfur consists of 8 sulfur atoms (S8) in a puckered ring structure; so it has a simple molecular structure | 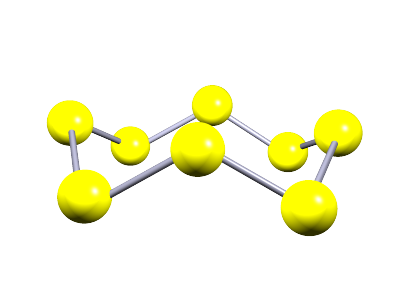 |
| Chlorine (Cl2) | Cl | −101 | Chlorine gas consists of small diatomic molecules (Cl2)-Simple molecular structure. |  |
| Argon | Ar | −189 | Argon like all noble gases is a monatomic gas; that is it consists of individual atoms with weak Van der Waals (London forces) between the atoms. |  |
The metals sodium (Na), magnesium (Mg) and Aluminium (Al) all have as you might expect giant metallic structures which consist of metal ions surrounded by a sea of delocalised electrons. Recall that metals atoms in their giant metallic structure lose their outer valency electron(s) which will form the sea of delocalised electrons and leave a metal ion. A metallic bond is formed by the attraction of these metal ions for the delocalised electrons.
Sodium being an alkali metal in group 1 of the periodic table will lose its outer 3s1 electron which will become delocalised through the giant metallic lattice structure of the sodium metal. This leave sodium ions (Na+) which will then form metallic bonds to these delocalised electrons in the metal lattice. In magnesium the two outer 3s2 electrons will be lost which will leave behind a magnesium ion with a 2+ charge. Now since there are twice as many delocalised electrons in magnesium compared to sodium and the magnesium ions have a larger charge and are also smaller than sodium ions this means that not only will there be more metallic bonding present in magnesium compared to sodium but it will be stronger since the delocalised electrons will be attracted to a smaller more highly charged magnesium ion; this is outlined in the image below:
| Sodium | Magnesium | Aluminium |
|---|---|---|
 |
 |
 |
| Sodium ions have a 1+ charge and only 1 electron per sodium ion is involved in forming the metallic bonds in the giant metallic lattice. | Magnesium ions have a 2+ charge and 2 electrons per magnesium ion are involved in forming the metallic bonds in the giant metallic lattice. | Aluminium ions have a 3+ charge and 3 electrons per aluminium ion are involved in forming the metallic bonds in the giant metallic lattice. |
Similarly with aluminium; being a group 3 metal means it will lose its outer 3s23p1 valence electrons to form an aluminium ion with a 3+ charge; this means that there will be more metallic bonds
 formed in aluminium than in magnesium or sodium and they will be much stronger since the aluminium ions are smaller and more highly charged than either the magnesium or sodium ions. This means that the melting points of the metals will increase as we move from sodium to magnesium to aluminium.
formed in aluminium than in magnesium or sodium and they will be much stronger since the aluminium ions are smaller and more highly charged than either the magnesium or sodium ions. This means that the melting points of the metals will increase as we move from sodium to magnesium to aluminium.
You might expect aluminium to have a much higher melting point than magnesium because it has a higher ionic charge (Al3+ compared to Mg2+), a smaller ionic radius, and more delocalised electrons in its giant metallic lattice. These factors all increase the strength of the metallic bonding. However, the melting point of aluminium is only slightly higher than that of magnesium. This is because the metallic lattice in aluminium has a different arrangement, and the greater number of delocalised electrons causes increased electron–electron repulsion, which offsets some of the extra attraction between the metal ions and delocalised electrons. As a result, the increase in metallic bond strength and melting point is smaller than might be expected.
Silicon has the highest melting point of the period 3 elements because it forms a giant covalent lattice with lots of strong Si-Si covalent bonds present, the giant covalent network structure of silicon is very similar to the diamond structure formed by carbon atoms. In this structure, each silicon atom is covalently bonded to four other silicon atoms in a tetrahedral arrangement, forming a continuous three-dimensional network. A large number of strong covalent bonds extend throughout the entire giant structure and breaking all these strong covalent bonds would require a large amount of energy; so as a consequence silicon has a very high melting point.
Interestingly although silicon has a structure similar to diamond, it cannot form a graphite-like allotrope. Recall that in graphite each carbon atom forms three strong sigma bonds and uses its remaining p orbital to form delocalised pi bonds across the flat layers of carbon atoms. However silicon’s outer 3p orbitals are much larger and more diffuse than carbon’s 2p orbitals, leading to poor sideways overlap and weak pi bonding, so silicon cannot form the delocalised layered structure found in graphite.
The next three elements in period 3 are the non-metal elements phosphorus, sulfur and chlorine. All these elements have small molecular structures with weak intermolecular Van der Waals (London dispersion forces) between the molecules and as a consequence they all have low melting points.

The discovery of phosphorus is a rather odd and curious tale; in 1669 the German alchemist Hennig Brandt (sometimes spelled Brand) was trying to make the Philosopher’s Stone; a mythical substance believed to turn base or imperfect metals such as iron, lead or copper into "noble metals" such as gold or silver. It was also thought to be an elixir of life which if consumed could grant immortality and cure any disease. Brandt believed that human urine, being golden in colour might contain the secret to creating the Philosopher's stone.
Brandt collected huge volumes of urine; reports suggest he collected over 4000 litres of urine which he then let stand until it gave off a strong odour. He then spent weeks boiling the urine he had collected down to a thick syrup and heated this residue with sand in a furnace. During this process, a white, waxy substance condensed on the cooler parts of the equipment. This new substance glowed in the dark for many hours, burned brightly, and smelled of garlic. Brandt had accidentally discovered phosphorus.
The common allotropes of phosphorus are:
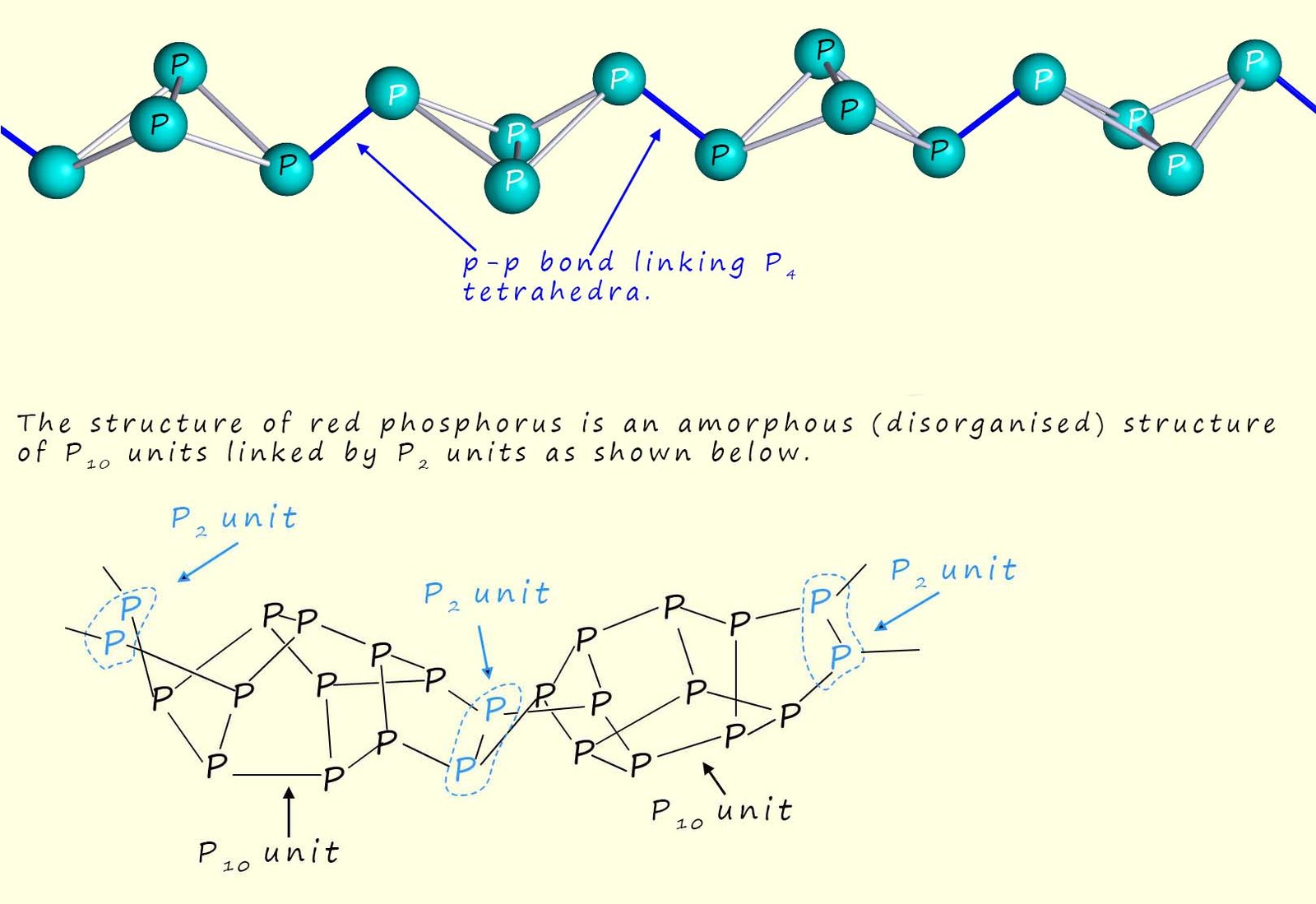
When heated strongly red phosphorus reacts with oxygen to form phosphorus pentoxide (P4O10) in a highly exothermic reaction that releases a large amount of heat energy. This property makes red phosphorus it useful in matches, fireworks and pyrotechnics where it acts as a fuel and ignition aid.
 In safety matches for example the red phosphorus is found on the striking surface/sandpaper strip found on the side of the box. This sandpaper strip also contains a very small amount of powdered glass; to supply additional friction when the match is struck. When the match is struck, friction converts a small amount of red phosphorus on the sandpaper strip into white phosphorus vapour, which immediately ignites in air. The heat from this ignition then triggers the decomposition of the oxidising agent potassium chlorate which is found in the match head. Now potassium chlorate is a powerful oxidising agent that decomposes according to the equation below:
In safety matches for example the red phosphorus is found on the striking surface/sandpaper strip found on the side of the box. This sandpaper strip also contains a very small amount of powdered glass; to supply additional friction when the match is struck. When the match is struck, friction converts a small amount of red phosphorus on the sandpaper strip into white phosphorus vapour, which immediately ignites in air. The heat from this ignition then triggers the decomposition of the oxidising agent potassium chlorate which is found in the match head. Now potassium chlorate is a powerful oxidising agent that decomposes according to the equation below:
2 KClO3 → 2 KCl + 3O2
This decomposition reaction produces oxygen gas, which supports and intensifies the combustion of other materials in the match head, such as sulfur or antimony(III) sulfide, which act as the fuels for the combustion reaction taking place. The extra oxygen ensures that the fuel and flame burn steadily, even though the match head is small and might otherwise not get enough oxygen from the air.
Similarly in fireworks the red phosphorus acts mainly as a fuel and ignition component rather than as a source of colour or light effects. Its key roles are as an Ignition aid. The red phosphorus burns easily once heated and reacts exothermically with oxygen to form phosphorus pentoxide (P4O10) exactly as occurred in the match. The heat from this reaction then ignites other chemicals inside the firework. The oxidation of the red phosphorus releases a large amount of energy, raising the temperature of the reacting mixture in the firework and ensures complete combustion of other fuels and metal powders present. The intense heat also helps decompose oxidising agents such as potassium chlorate or potassium perchlorate, which release oxygen which rapidly increases the rate of combustion of the main reactions taking place in the firework.

After phosphorus the next two elements in period 3 are sulfur and chlorine. Both of these elements have small molecular structures. A common allotrope of sulfur is called rhombic sulfur or α-sulfur, it is a brittle yellow solid at room temperature and it has a small molecular structure compose of 8 sulfur atoms in a puckered ring structure as shown below. While chlorine gas is composed of small diatomic molecules, that is molecules consisting of only two atoms. The structure of these two molecular substances is shown below:
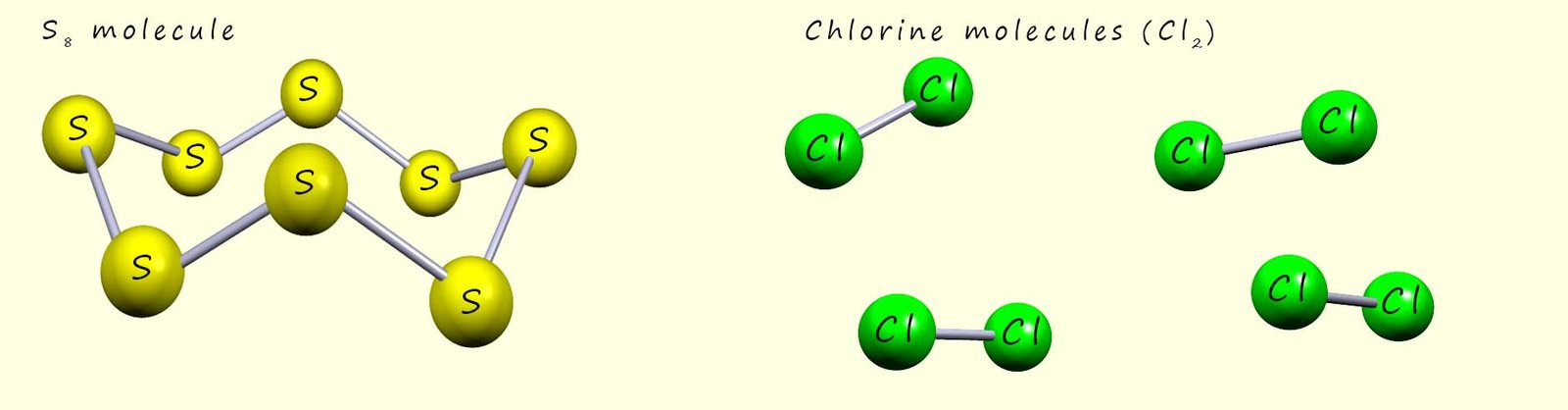

Sulfur melts easily when heated on a burning spoon using a Bunsen burner at around 1150C to form a red-brown tacky liquid, if heating continues a small blue coloured flame will be seen as the sulfur burns to form the choking, toxic and eggy-smelling gas sulfur dioxide (SO2).
Chlorine (Cl2) has a melting point of -101.50C; the reason why sulfur, chlorine and phosphorus all have low melting points is simply because they all consist of small non-polar molecules with weak Van der Waals forces (London dispersion forces) between the molecules.
These Van der Waals (London dispersion forces) forces are temporary, weak attractions that arise from the random movement of electrons creating instantaneous, temporary dipoles in the molecules. Since these intermolecular forces are very weak little energy is needed to overcome them and allow the molecules to move freely in the liquid state, this is why their melting points are so low.
Larger molecules or atoms with more electrons have more easily polarised electron clouds, this produces stronger temporary dipoles and therefore stronger van der Waals’ forces (London dispersion forces) between molecules or atoms and as a result, more energy is needed to overcome these forces during melting. Since sulfur molecules are larger than phosphorus molecules and have more electrons they will have more and also stronger Van der Waals forces between them and so sulfur will have a higher melting point than phosphorus. The same reasoning can be used to explain why phosphorus has a higher melting point than chlorine. We can summarise this in the table below, I have also included data on the noble gas argon which we will discuss further below:
| Substance | Approx. electrons per molecule | Relative van der Waals’ strength | Melting point |
|---|---|---|---|
| Ar | 18 | Weakest | Very low |
| Cl2 | 34 | Weak | Low |
| P4 | 60 | Moderate | Higher |
| S8 | 128 | Strongest | Highest |
Recall that the noble gases are all unreactive monatomic gases, that is they consist of individual atoms. This means that the only forces present between the atoms of any noble gas will be Van der Waals (London dispersion forces) forces and since the strength of the intermolecular Van der Waals bonding depends on the size (surface area) of the atom and also the number of electrons present then the strength of the Van der Waals forces in noble gases will be very weak indeed, this explain why argon with a melting point of -1890C has the lowest melting point of any period 3 element.
The graph below provides a useful visual summary of the trends in the melting points for the period 3 elements along with the structures for these elements.
Simply use the move up and move down buttons below to place the period 3 elements in order of decreasing melting point; press the check answer button when your done.