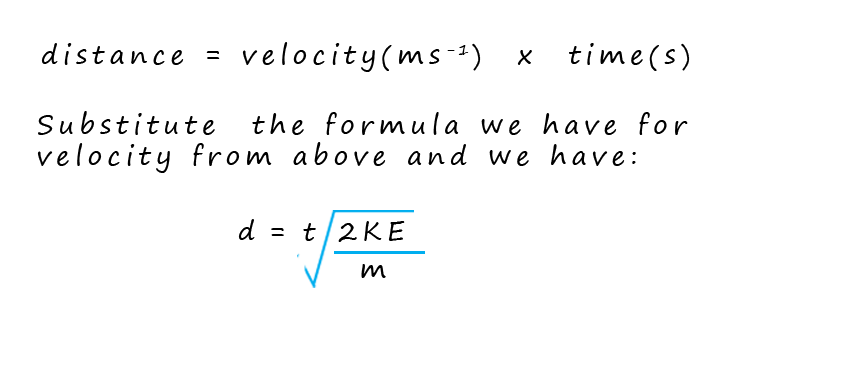A mass spectrometer is used to identify unknown substances, to determine the composition of mixtures and to
accurately measure the relative amounts and masses of substances present. It can for example be used to find the masses and abundances of isotopes present in an element and from this it is relatively straightforward to calculate the relative atomic mass of the element. The mass spectrometer also has many uses in the chemical, engineering
and space industries, for example the Rovers which NASA sent to the planet Mars all had mass spectrometers on board to
analysis Martian rocks, minerals and gases in the atmosphere.
In the A-level chemistry specifications there are two main types of mass spectrometers that you will need to
know about. If you are studying the AQA specification you will need to be able explain the working of a time
of flight mass spectrometer and carry out some calculations based on this method of mass spectroscopy. So
let's start here. For details on the other type of mass spectrometer, the single beam mass spectrometer and its
operation click here or use the link above.
The time of flight mass spectrometer was developed during the 1950. These mass spectrometers are smaller, less expensive and more compact than traditional single beam mass spectrometers. To analyse the sample of the unknown substance under examination we need to consider what happens in each of 4 separate key stages in the operation of this type of mass spectrometer. These 4 stages are:

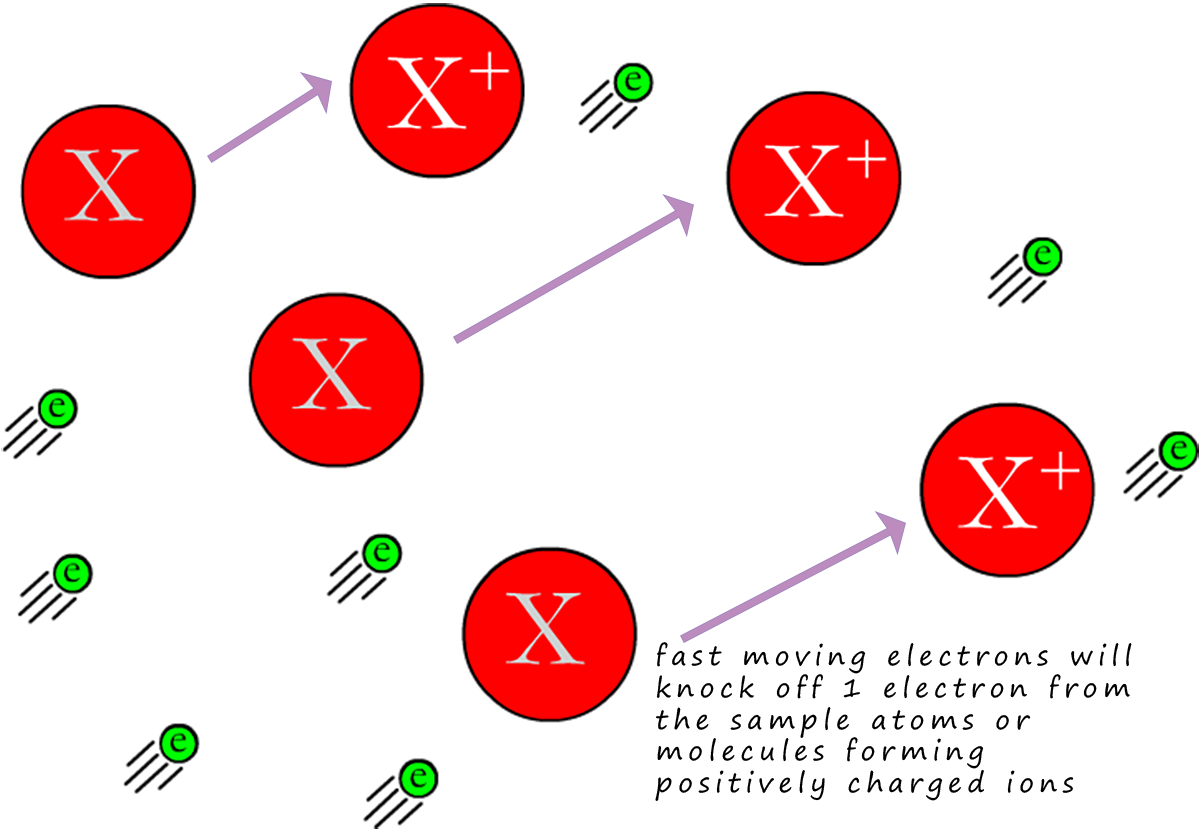
If we imagine the electrons as being like fast moving solid particles
or balls/bullets; then when the molecules
or atoms in the injected sample meet these fast moving electrons, they will
have 1 of their outer
electrons removed or knocked off, this means they will have been ionised.
This will leave a positively charged ion behind, now almost all of the ions produced will have a +1 charge (this is shown in the image opposite). When the sample under test is ionised in this way to form ions with a +1 charge the ion formed is often referred to as the molecular ion.
 If we call the injected sample X, then we can show this as:
If we call the injected sample X, then we can show this as:
Where e represents the electron being knocked off the sample X. You may also see this equation written as:
Here the first e represents the electron from the electron gun which impacts the sample X, forming a positively charged ion ( X+(g)) and the 2e on the product side represent the one electron lost by the sample X and also the electron from the electron gun.
For example the equations below represent the ionisation of neon gas and hydrogen gas:
And for hydrogen gas we have:

The second method of ionising the sample uses a method called electrospray ionisation. This method is often preferred for larger molecules which may be blasted to pieces by an electron gun while electrospray ionisation rarely results in fragmentation. Here the sample is dissolved in a volatile polar solvent (volatile means it evaporates easily) such as water or methanol, next it is injected into the sample point under high pressure and through a very fine hypodermic needle which has a high voltage (around 4000V) applied to it. This results in the sample being ionised and forming fine droplets or a mist containing the ionised sample. The solvent being volatile quickly evaporated leaving the positively charged sample. This is shown in the diagram below
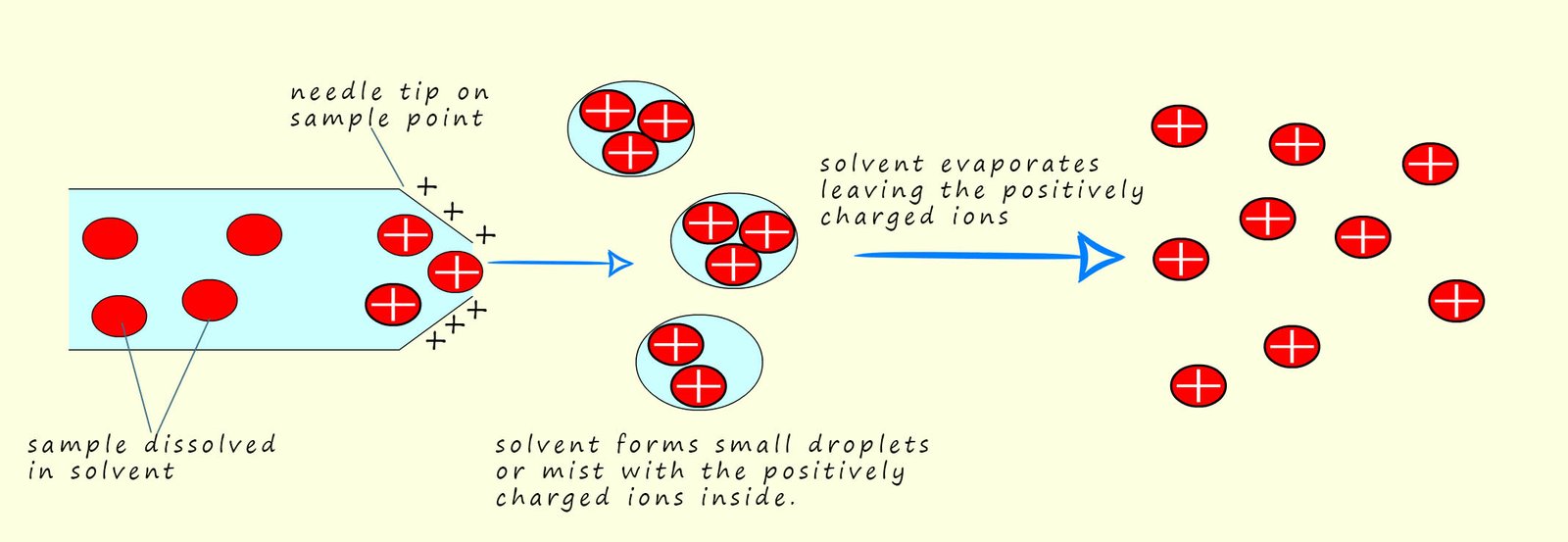
The sample X becomes ionised not by having electrons knocked off it or removed from it as is the case in electron impact ionisation, instead the sample under test gains a hydrogen ion (H+) from the solvent. This means that the mass of the sample molecule will increase by 1 unit due to the addition of a hydrogen ion. We can show this as:
All this takes place in the needle on the end of the injection point. This is also shown below:
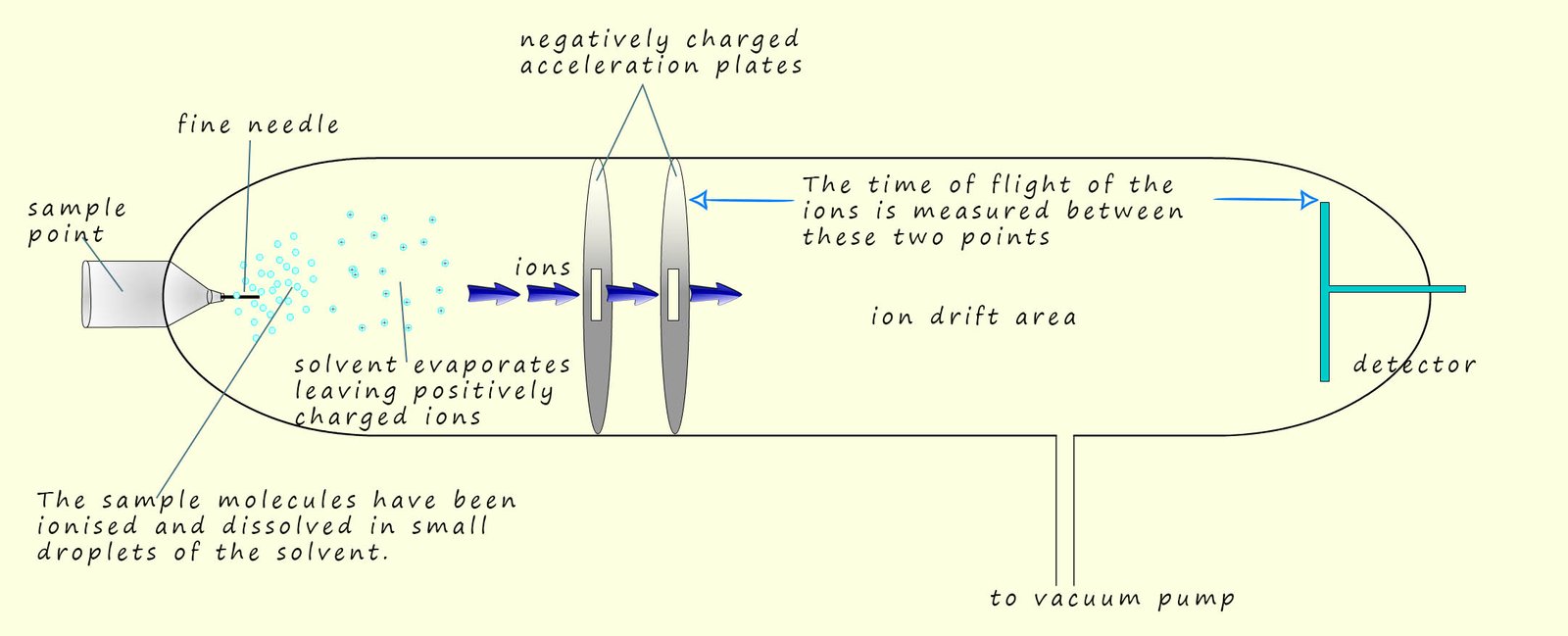
The next key step in the mass spectrometer is
acceleration of the ions formed.
There is one vital assumption that we will make: we will assume that all the
ions formed in the ionisation step have
a charge of +1. The positively
charged ion will be now be accelerated
up the tube by an electric field, they will be attracted to and accelerated towards the negatively charged plates.
One of the main pieces of information that the mass spectrometer provides is the
Ar or Mr of the substance under analysis. If the sample contains a mixture of
substances it would be very useful if we could obtain the Mr for each substance present.
Now after the ionisation step we may have a range of different substances
all with a charge
of +1. The mass spectrometer will give you a read out of the mass to charge ratio,
often called m/z ratio of any substance it detects. Now if the charge (z) is simply
equal to +1, then the mass to charge ratio (m/z) will simply be the mass.
In the acceleration stage in the mass spectrometer
would you expect the heavy or
light ions to be accelerated quickest up the tube? Or put another way, if you
pushed a golf ball or a bowling ball, which would move with the greatest
velocity or speed? Obviously the lightest ions will be accelerated quickest
up the tube towards the charged plates.
This is outlined in the image below, here three isotopes of the metal magnesium are shown, 24Mg+, 25Mg+ and 26Mg+. All three magnesium ions will have the same kinetic energy after the acceleration stage but the lighter 24Mg+ ions will have the largest velocity and will therefore reach the detector first, whereas the heavier and slower 26Mg+ ions will be last to reach the detector.
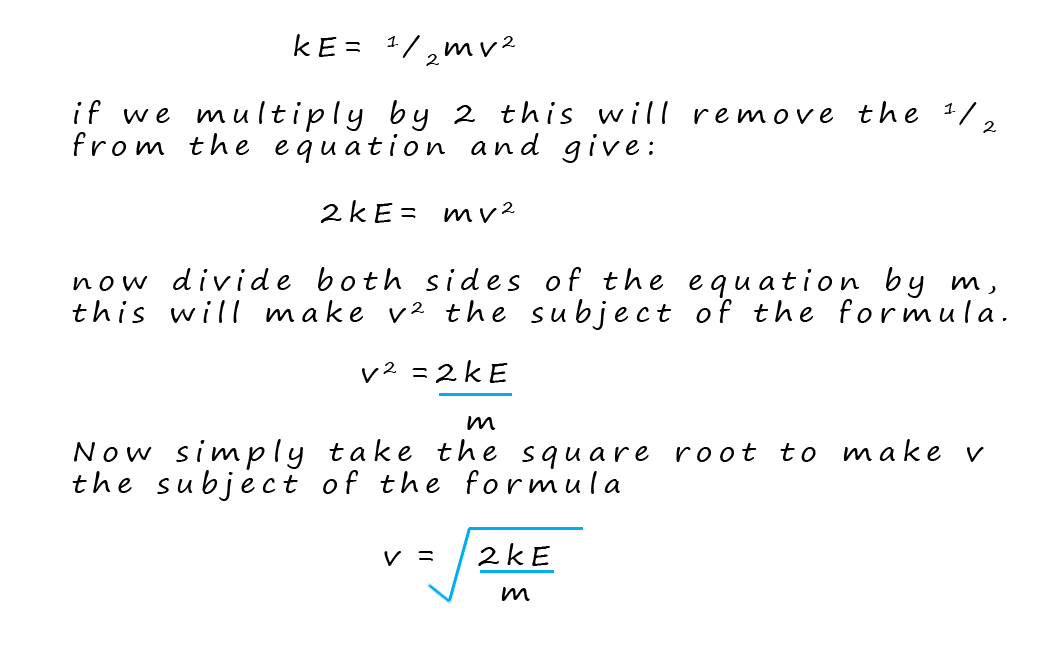
 Now you may remember from your
physics lessons that the kinetic energy of an object can be calculated
from the formula:
Now you may remember from your
physics lessons that the kinetic energy of an object can be calculated
from the formula:
Where: m =mass in kilograms (kg), v= velocity in metres per second (ms-1) and the KE = kinetic energy in joules (J).
A key feature of this type of mass spectrometer is that all
ions, no matter their mass
will all gain the same amount of kinetic energy during the
acceleration stage. The
kinetic energy of the ions
will depend on their mass and velocity, so lighter faster
moving ions will have the same kinetic energy as heavier slower moving ions.

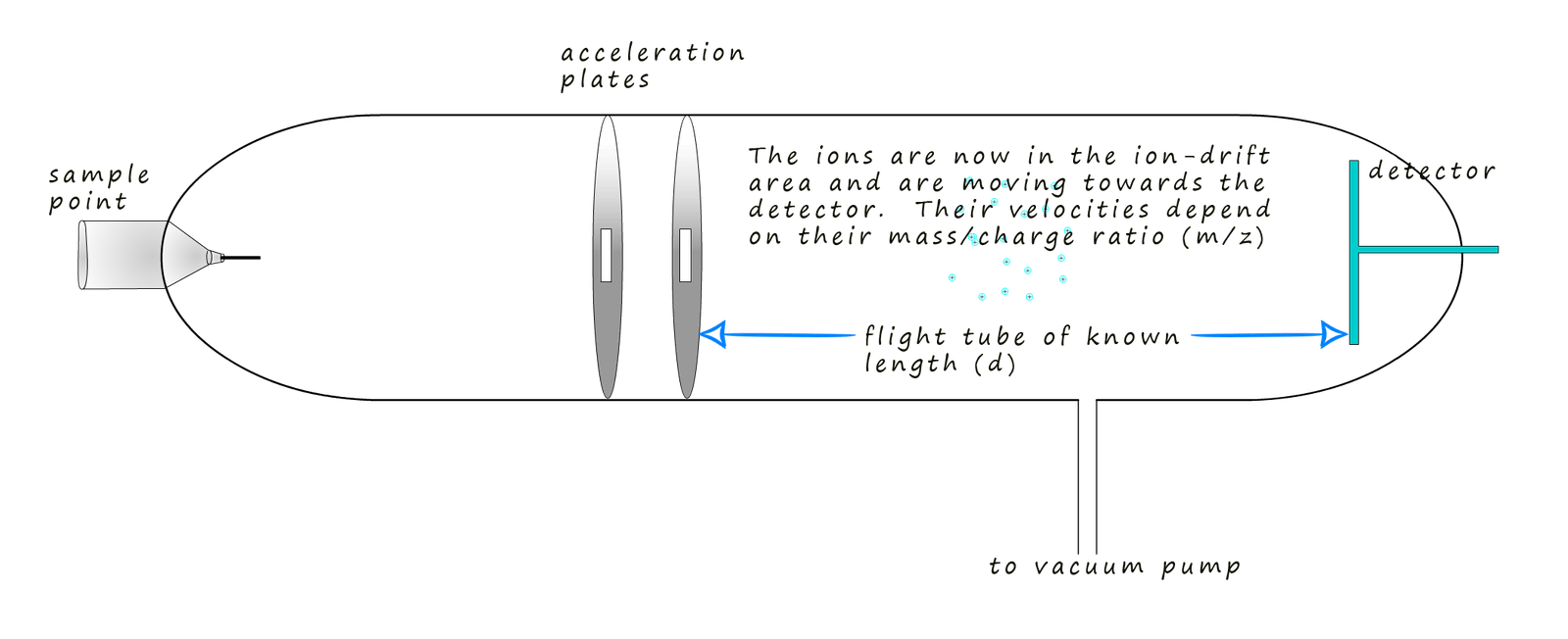
The detector plate at the end of the flight tube has a negative charge due to an excess
electrons being placed on it from an electrical source, now
when the positively charged ions
arrive at the detector plate they gain an electron
and are reduced.
This reduction reaction involves the movement of electrons, or a flow of electrical current. The
more electrons that flow the more
ions are present; that is their abundance is high, this will be recorded by a
computer connected to the detector. Remember the ions
will arrive at the detector plate at various times since their
flight time down the flight tube will obviously depend on how fast
they are travelling and this as previously
mentioned depends on their mass to charge ratio; or simply their mass if the charge on the ion is +1.
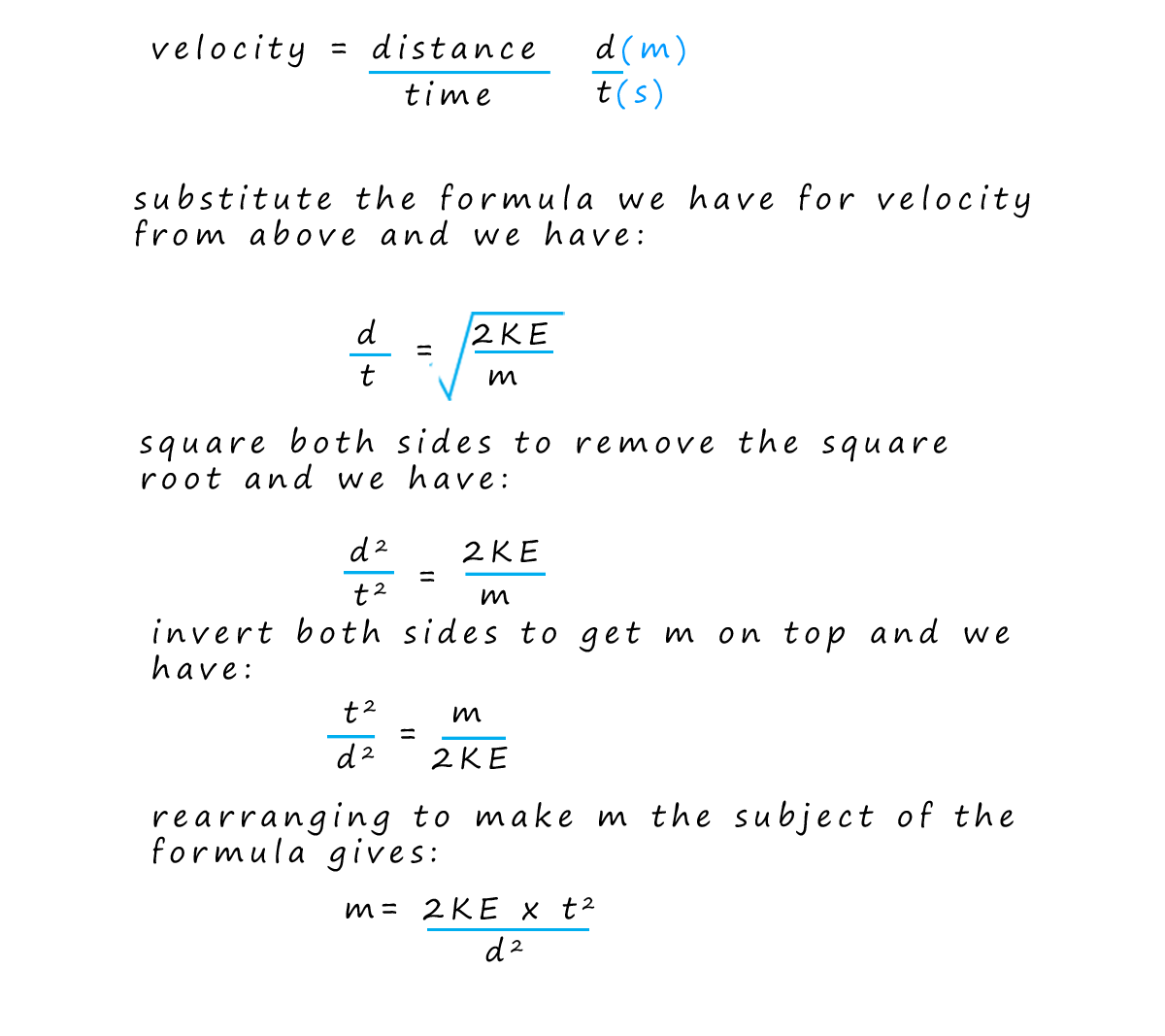
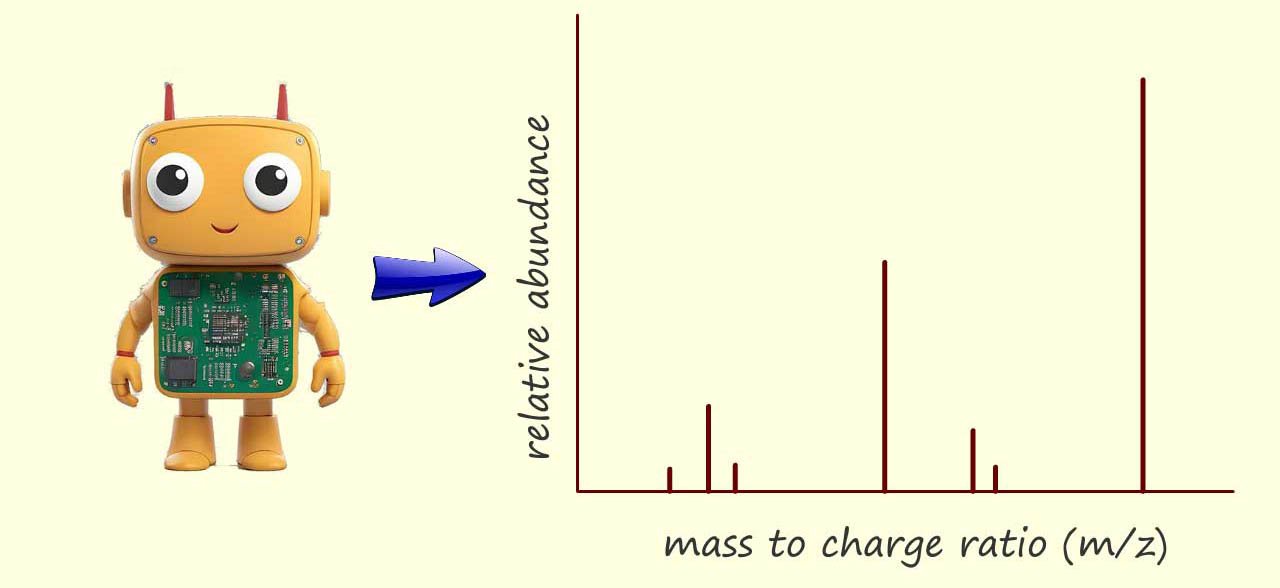 By knowing the distance the ions travelled (that is the fixed length of the flight tube) and measuring their arrival times; the computer can calculate the velocity of each ion and using the equation for kinetic energy (where the kinetic energy is constant for all ions due to the acceleration stage) the computer can then determine the mass-to-charge ratio (m/z) of each ion that hits the detector. Finally the software creates a mass spectrum. This spectrum is a plot of the m/z values on the x-axis versus the intensity (proportional to the number of ions) on the y-axis. The mass spectrum provides a visual representation of the different ions present in the sample and their relative abundances.
By knowing the distance the ions travelled (that is the fixed length of the flight tube) and measuring their arrival times; the computer can calculate the velocity of each ion and using the equation for kinetic energy (where the kinetic energy is constant for all ions due to the acceleration stage) the computer can then determine the mass-to-charge ratio (m/z) of each ion that hits the detector. Finally the software creates a mass spectrum. This spectrum is a plot of the m/z values on the x-axis versus the intensity (proportional to the number of ions) on the y-axis. The mass spectrum provides a visual representation of the different ions present in the sample and their relative abundances.
In the exam your exam you could be asked to calculate any one from the list below. It is often necessary to rearrange formulae, I have given some examples below but I would try the practice questions and complete as many of the examples as possible to ensure you are completely confident with these questions.
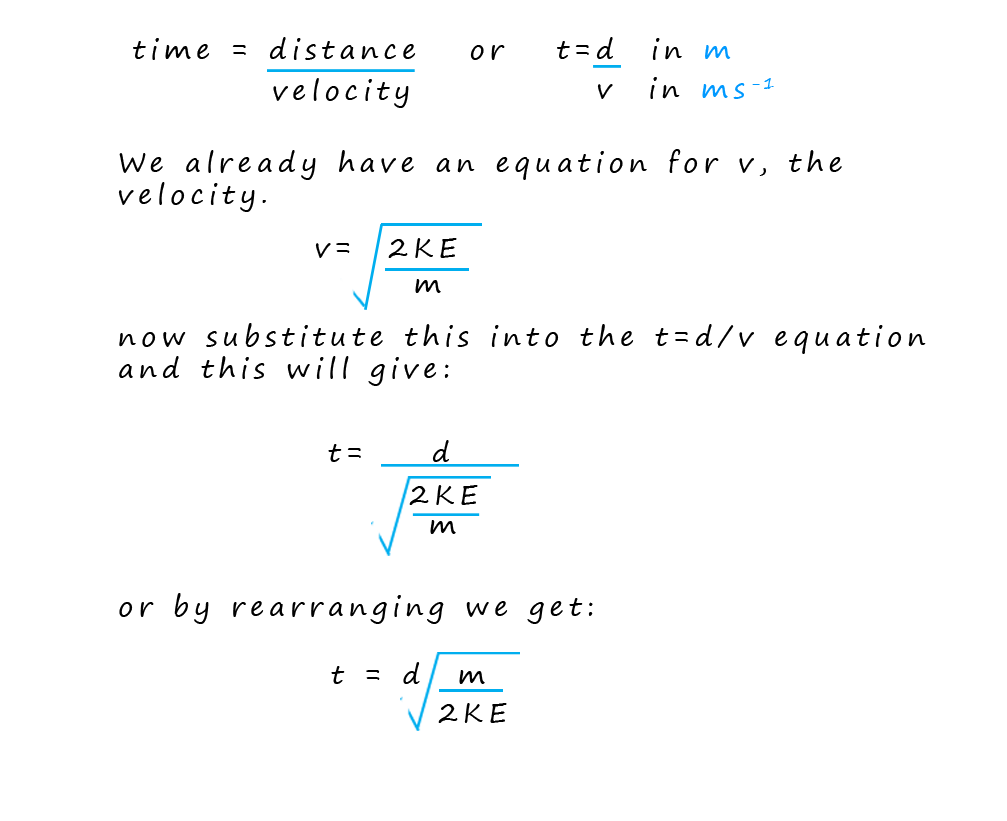
This equation tells us that time of flight (t) is proportional to the square root of the mass (√m), so lighter ions arrive at the detector before heavier ones.