

Note: If your are not sure about bond dipoles and electronegativity then it would be wise to review this before reading this page on dipole moments.
 If a molecule contains polar covalent bonds then the bonds will contain a bond dipoles; that is the molecule will contain atoms
with partial positive charges (δ+) and partial negative charges (δ-). As long as the molecule is non-symmetrical then these bond dipoles will result in the
molecule being a polar one. If
the molecule is highly symmetrical it is likely the bond dipoles
will all cancel each other out due to the geometry and shape of the molecule, this
means that the molecule will be non-polar.
If a molecule contains polar covalent bonds then the bonds will contain a bond dipoles; that is the molecule will contain atoms
with partial positive charges (δ+) and partial negative charges (δ-). As long as the molecule is non-symmetrical then these bond dipoles will result in the
molecule being a polar one. If
the molecule is highly symmetrical it is likely the bond dipoles
will all cancel each other out due to the geometry and shape of the molecule, this
means that the molecule will be non-polar.
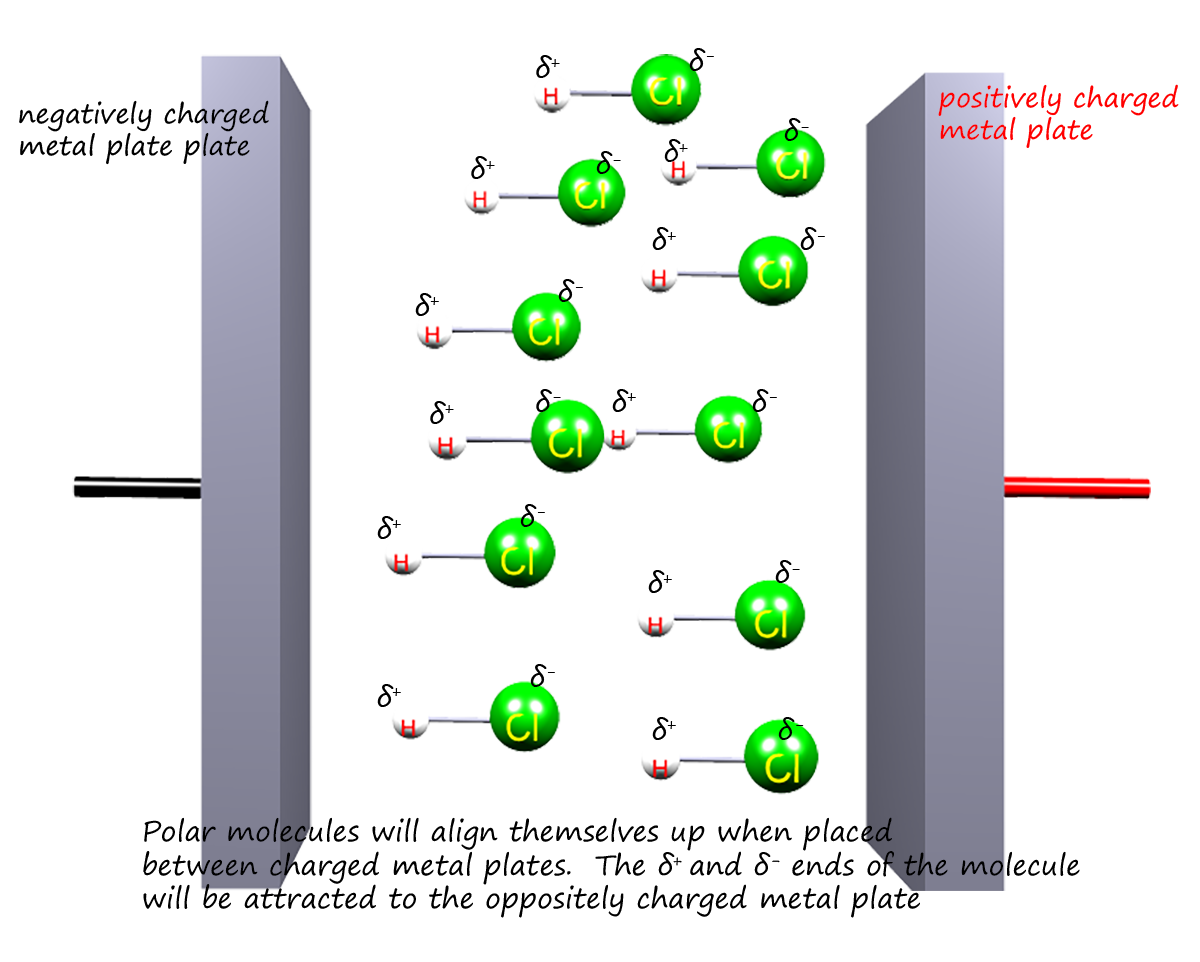
If a polar molecule is placed between two electrically charged plates, as shown opposite, then the molecules
will align themselves up in such a way that the partially
positively charged (δ+) end of the polar molecule will align
with the negatively charged plate and the δ-
end of the molecule will align itself with the positively charged plate. Non-polar molecules
will simply arrange themselves in a random way if
placed between the two charged plates.
The overall net polarity of a molecule is called the dipole moment. It is given the Greek symbol µ (pronounced mu).
The dipole moment of a
molecule depends on the magnitude (size) of the charges
at the end of the polar bond and on the distance between these
two oppositely charged atoms in the polar covalent bond.
As an example consider the hydrogen halides: HF, HCl, HBr and HI; these are shown in the table below. You can
clearly see
that as the difference in electronegativity increases, which means a
larger partial positive charge (δ+) on the hydrogen
atom a negative partial charge (δ-) on the halide atom,
then the dipole moment increases in size. It is worth mentioning that all these molecules are non-symmetrical
and have
a permanent bond dipole and therefore the molecules will be polar.
| Substance | structure | difference in electronegativity | dipole moment |
|---|---|---|---|
| hydrogen fluoride | 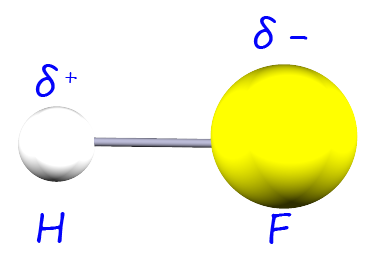 |
4.0-2.1= 1.9 | 1.8 |
| hydrogen chloride | 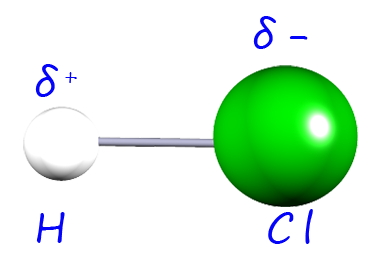 |
3.0-2.1= 0.9 | 1.1 |
| hydrogen bromide | 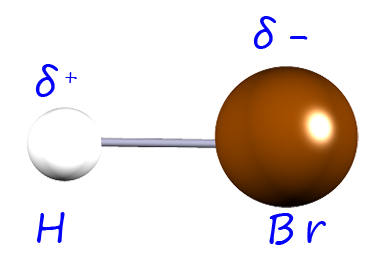 |
2.9-2.1= 0.8 | 0.8 |
| hydrogen iodide | 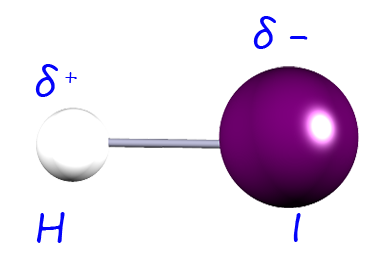 |
2.6-2.1= 0.5 | 0.44 |
The dipole moment of a molecule is calculated using the formula:
µ= Q x r
Where:
You may have carried out a practical investigation in class where you can compare the size of the
dipole moment
present in various liquids simply by measuring how far and at what angle various liquids are
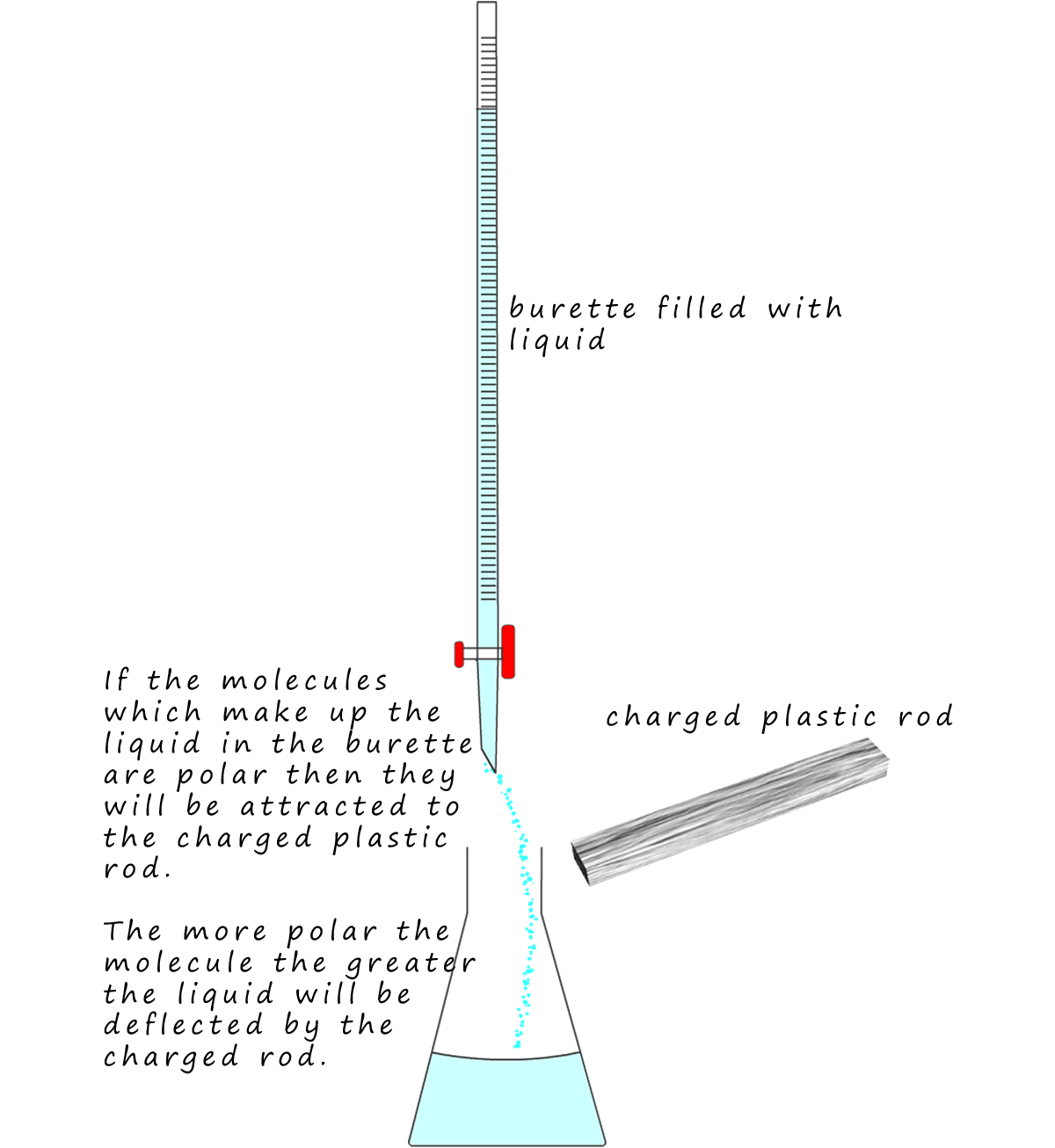
deflected by a charged rod.
The set-up is very simple. The liquids under test are placed
in a burette. The tap in the
burette is opened and a slow stream of liquid
is allowed to flow out. Next the plastic
rod is charged by rubbing it with a dry cloth or fur rag.
The rod will become either
positively or negatively charged. The charged rod is then
brought up to the slow flowing
liquid from the burette and using a ruler you can estimate
how far the liquid is
deflected. This is shown opposite.
We can offer an explanation as to why some liquids will be deflected by the charged rod. In the example below the first 2 burettes are filled up with water. Water is a polar molecule with a dipole moment. This means that when the charged rod is placed near the stream of water, the water molecules will orientate themselves in such a way that the oppositely charged portion of the molecule is directed towards the charged rod. If you look at the diagram closely you will see that the water molecules are orientated differently in each of the first 2 water streams from the burette. This is simply because the charge on the rod in each example has been changed. In the final example it does not matter whether you use a rod with a positive or negative charge. Tetrachloromethane is a non-polar molecule and will not be attracted to the rod; no matter what charge is on it.
