
Higher and foundation tiers
This page builds on the ideas discussed in the pages on bases and alkalis, if you need a quick re-cap on the difference between acids and bases and neutralisation reactions then click here.
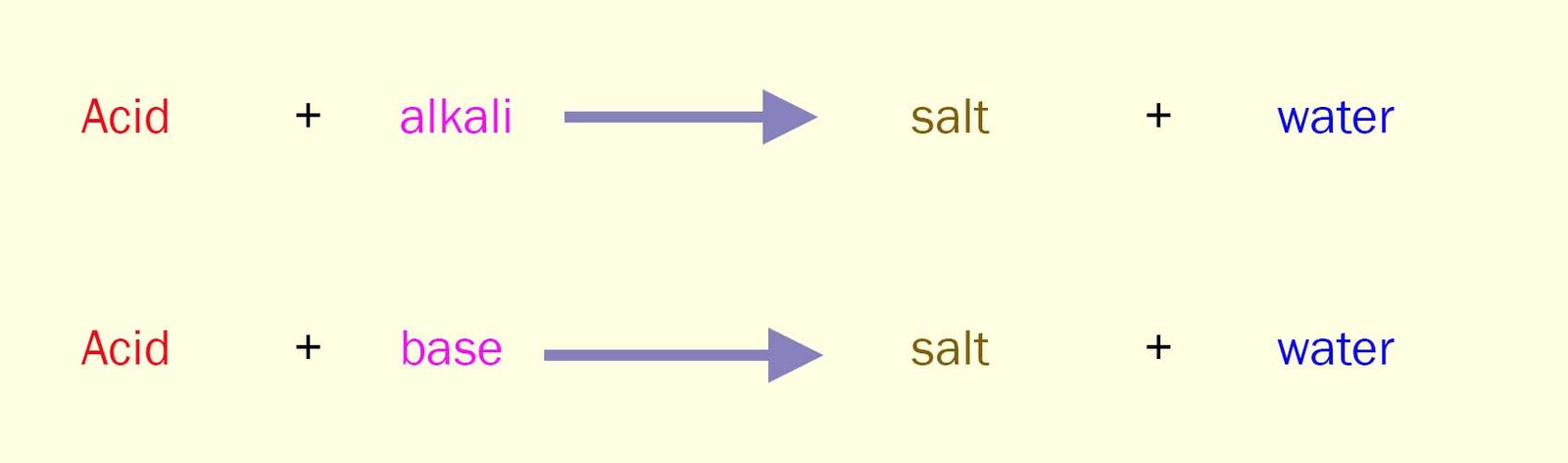
You can neutralise an acid using an alkali or a base. The equations for these neutralisation reactions can be written as:

 Bases are substances which can neutralise an acid.
They react with acids to produce a salt
and water.
This is identical to the reaction of alkalis with acids.
But remember alkalis are aqueous solutions;
they are formed when a base dissolves in water.
Bases on the other hand are mostly solids.
Bases are substances which can neutralise an acid.
They react with acids to produce a salt
and water.
This is identical to the reaction of alkalis with acids.
But remember alkalis are aqueous solutions;
they are formed when a base dissolves in water.
Bases on the other hand are mostly solids.
Common bases include metal oxides, metal carbonates and metal hydroxides. Indigestion tablets
for example contain metal carbonates; commonly 0.5g of calcium carbonate (chalk). Calcium carbonate is an excellent
base and will quickly neutralise any
excess acid present in the stomach and relieve the symptoms of acid
indigestion.
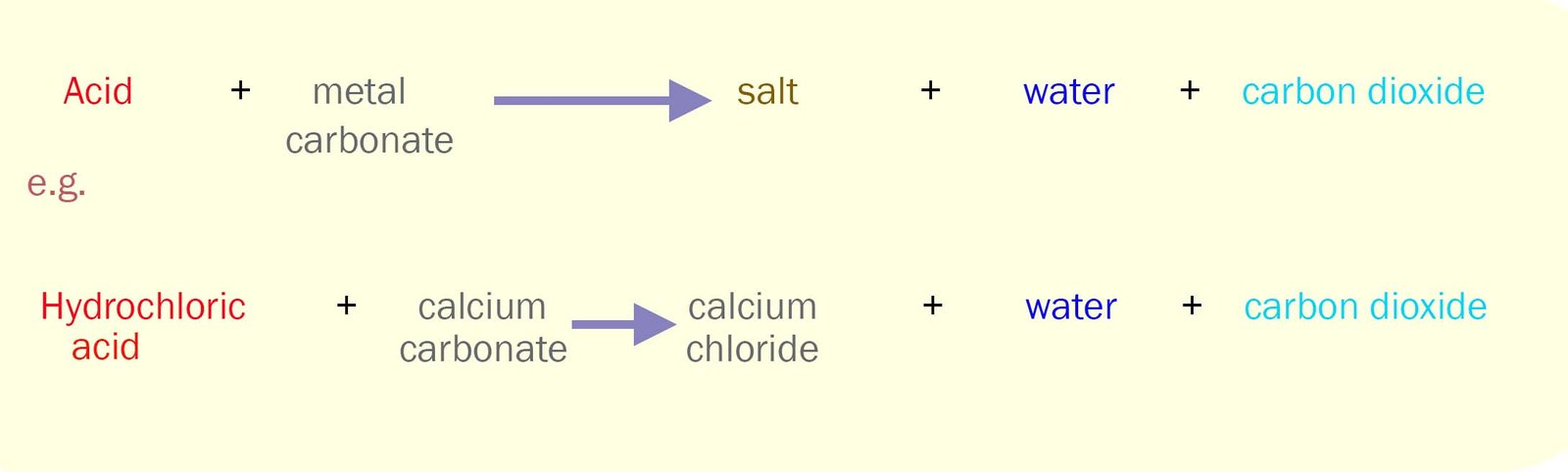
Metal carbonates such as calcium carbonate react with acids
according to the equations shown in the box below. The carbon dioxide gas which is given off is the reason why some people may feel slightly
bloated after taking an indigestion tablet.
Some bases will dissolve in water; however most are insoluble and will not dissolve in water. If a base dissolves in water it will form an alkali; that is a solution which contains an excess of hydroxide ions (OH-).
Drag the statements on the left to the correct definitions found in the boxes on the right hand side.
Most bases will not dissolve in water to form an alkaline solution since they are
insoluble.
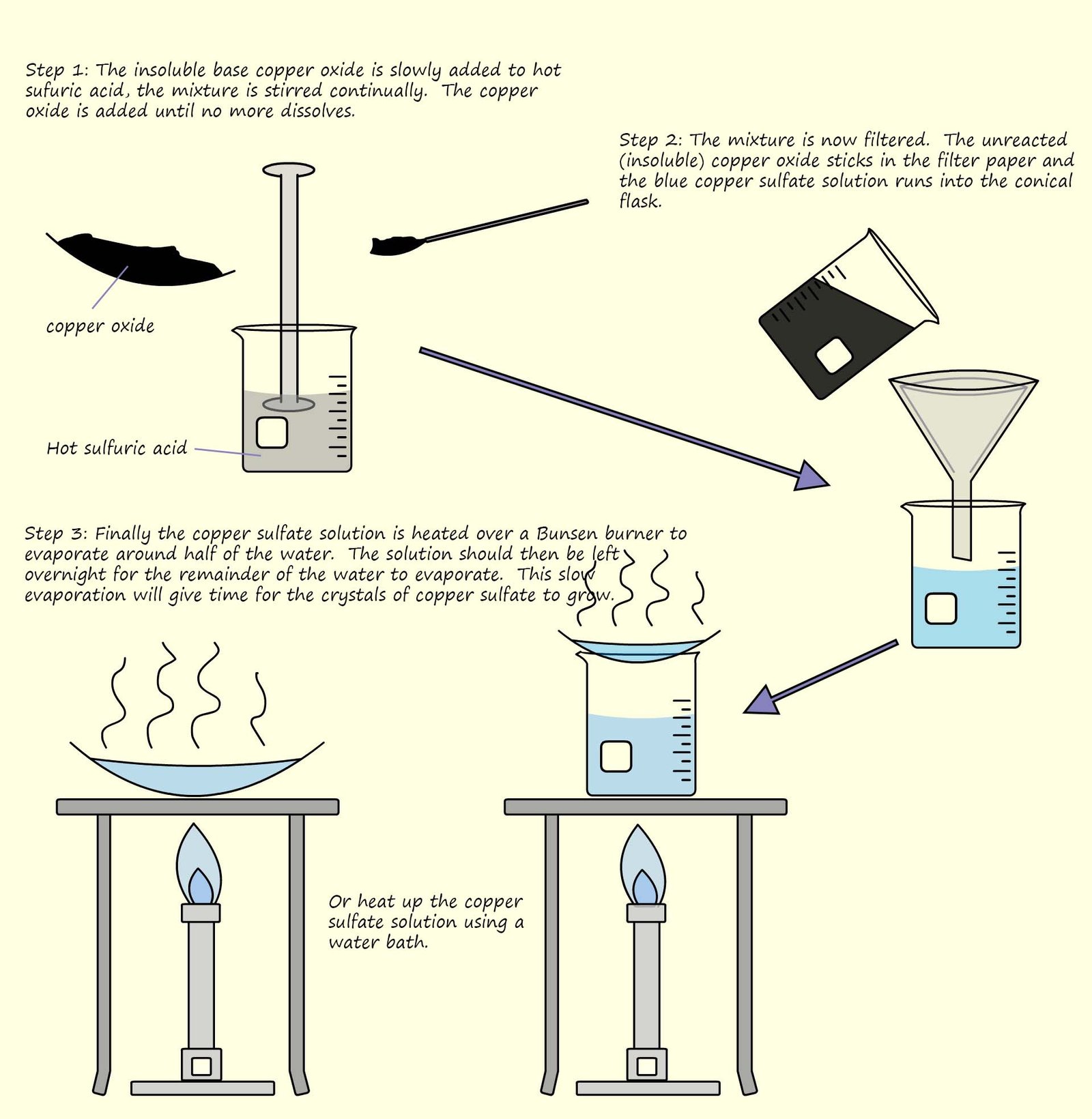
However they will reacts with acids. In the example below the insoluble base copper oxide is slowly added to a beaker
containing warm sulfuric acid.
The copper oxide reacts with the hot
acid; the basic copper
oxide is slowly added to the acid and stirred until it no longer dissolves, this indicated that all of the acid has reacted and the copper oxide will not dissolve in the remaining aqueous solution.
If the copper oxide no longer dissolves then this means all the acid has been neutralised.
The mixture is then filtered to remove the excess or unreacted copper oxide and finally the solution is heated to evaporate the water leaving behind the solid
salt.
The experimental procedure is outlined in the diagram below and
an equation for the neutralisation reaction taking place is shown below:
The blue copper sulfate solution is heated using a water bath or you could use an electric heater. This will leave a white solid in the evaporating basin; this white solid is anhydrous copper sulfate (anhydrous simply means without water). Recall that copper sulfate crystals if dry are white or colourless but if they are left in an open room the crystals absorb water from the air and turn blue, this form of copper sulfate is called hydrated copper sulfate. If the blue hydrated copper sulfate crystals are heated the water evaporates again and you are left with colourless (white) anhydrous copper sulfate crystals.
A simple way to determine the purity of any solid crystalline material is to measure its melting point and then compare it to the published or expected melting point of the solid crystalline substance. In order to measure the melting point of any solid crystals they will need to be washed and recrystallised before any melting point determination can take place. The recrystallisation process removes impurities from the original sample; the process basically involves dissolving an impure solid in the minimum volume of a suitable solvent needed to dissolve it and then simply cooling the solution down by placing it in an ice-bath, this will force the dissolved solid out of solution and it will start to recrystallise or form solid crystals.
The solvent is gently heated and the impure substance is then dissolved in the warm solvent, the solution is then stirred and heated to evaporate some of the solvent. If any solid insoluble impurities are visible the solution is then filtered using the Buchner apparatus shown below to remove these impurities.
Next the solution is now cooled by placing it in a beaker of iced water, as it cools the solubility of the dissolved solute will decrease and it should "fall out" of solution as solid crystals, these crystals will be much purer than the original sample which was dissolved.

Any solid crystals obtained from a reaction such as the neutralisation reaction above or a precipitation reaction should be filtered using the Büchner funnel shown opposite. The Büchner funnel simply uses suction from a water pump to help dry any crystals you have. The procedure for using the Büchner funnel is outlined below:
The crystals should now be purified by recrystallisation them using the following procedure:
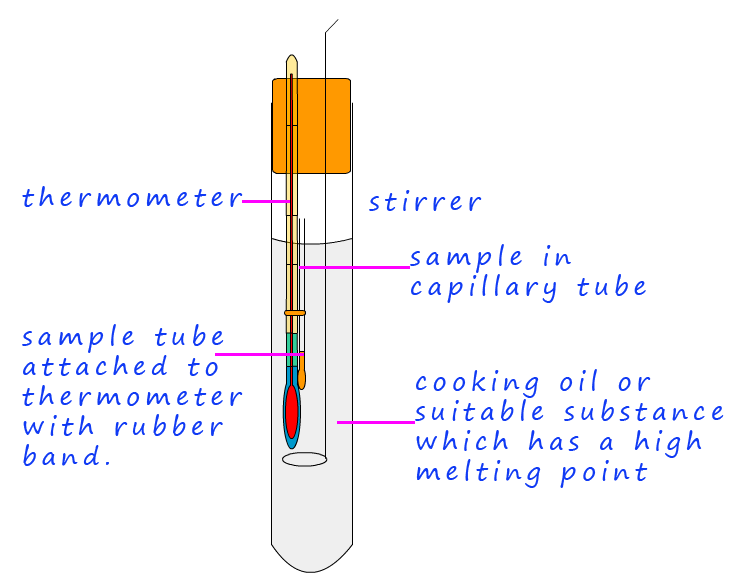
Finally to test the purity of the crystals they need to have their melting point taken. If the crystals or solid sample is pure it will have a sharp melting point, that is it will melt at the expected temperature, the presence of any impurities will mean that the substance under test may melt over a wide temperature range and the melting point will likely vary from the expected one. The following outlines the basic steps needed to carry out a melting point determination.

Test your understanding of the main ideas covered above by filling in the blanks to complete the paragraph below. The words to complete the blanks are shown in the yellow box and the drop down menus. Simply select the correct word or words from the drop-down menus to correctly complete the two short pargraghs.