

Higher and foundation tiers
 One of the essential skills you must have as a good chemist is
to be able to calculate how much of a particular reactant you will need to use to make a certain amount of your desired product or how much of a certain
product you will be able to make from your starting reactants.
The problem here is that it is not possible to count out or weigh individual atoms into a flask; since the atoms are by themselves far too small to weigh or count so counting atoms
is a complete non-starter.
One of the essential skills you must have as a good chemist is
to be able to calculate how much of a particular reactant you will need to use to make a certain amount of your desired product or how much of a certain
product you will be able to make from your starting reactants.
The problem here is that it is not possible to count out or weigh individual atoms into a flask; since the atoms are by themselves far too small to weigh or count so counting atoms
is a complete non-starter.
What is needed is a quantity or number that will let you compare
equal numbers of particles and use this number to calculate how much of the reactants to use or how much product you can expect to make in a any chemical reaction.
Chemists are not the only people faced with this problem; consider
paper! You don't buy individual sheets of paper; you buy paper in packs called reams. A ream
contains 500
sheets of paper. A ream of A3 paper will be bigger than a ream
of A4 paper because A3 paper is
bigger than A4 paper- obviously! But a ream of A3 paper will still contains 500 sheets of
paper.
The ream is a standardised unit used by paper suppliers because it contains a
standard number of sheets;
500 sheets. So if an order comes in for 10 000 sheets of paper would you rather count out
10 000 sheets of paper or quickly count out 20 reams?
 Chemists like paper suppliers
need a standard unit which they can use to measure out equal
numbers of reacting particles, whether they are atoms, molecules or ions. Now chemists don't use reams instead they use a unit
called the mole. The word mole may seem an odd choice of a word, since most people think of
moles as furry little critters that dig up your lawn! However the word mole is actually derived from a
Latin word meaning mass or pile, so think of the mole as the unit to work out the mass of reactants and products used or produced in chemical reactions. 1 mole of a substance is the mass of 6.022 x 1023 particles, that is Avogadro's number of particles.
So like the example above with paper, would you rather measure out 1 mole of a substance
or count out 6.022 x 1023 atoms?
Chemists like paper suppliers
need a standard unit which they can use to measure out equal
numbers of reacting particles, whether they are atoms, molecules or ions. Now chemists don't use reams instead they use a unit
called the mole. The word mole may seem an odd choice of a word, since most people think of
moles as furry little critters that dig up your lawn! However the word mole is actually derived from a
Latin word meaning mass or pile, so think of the mole as the unit to work out the mass of reactants and products used or produced in chemical reactions. 1 mole of a substance is the mass of 6.022 x 1023 particles, that is Avogadro's number of particles.
So like the example above with paper, would you rather measure out 1 mole of a substance
or count out 6.022 x 1023 atoms?
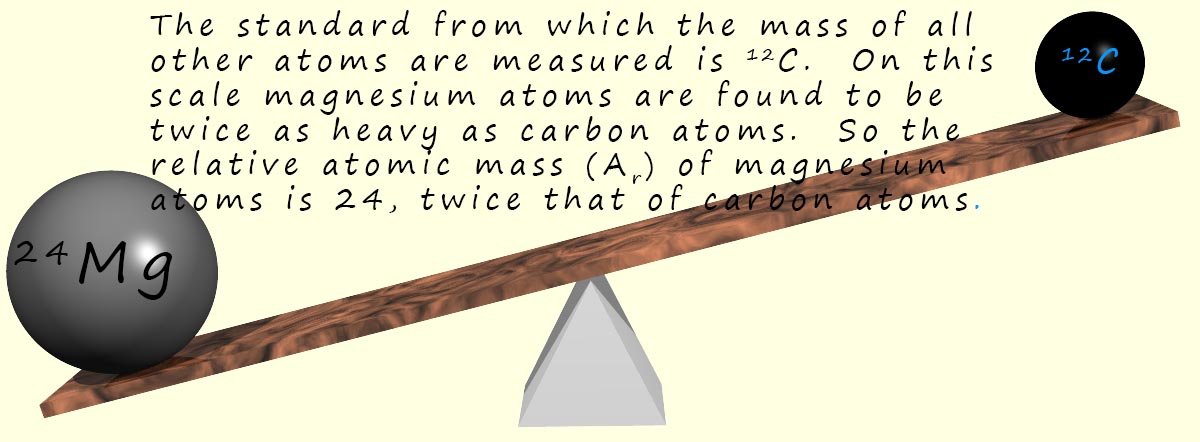 Chemists use a relative atomic mass scale to measure the mass of atoms
(relative simply means compared to). In 1961 the isotope of carbon (12C) was picked
as the standard from which the masses of all other atoms would be compared. On the carbon-12 scale
the 12C isotope is given an atomic mass of exactly 12 units. This means that 12 grams of 12C
contains 1 mole of carbon atoms. That is 6.022 x 1023 atoms.
Chemists use a relative atomic mass scale to measure the mass of atoms
(relative simply means compared to). In 1961 the isotope of carbon (12C) was picked
as the standard from which the masses of all other atoms would be compared. On the carbon-12 scale
the 12C isotope is given an atomic mass of exactly 12 units. This means that 12 grams of 12C
contains 1 mole of carbon atoms. That is 6.022 x 1023 atoms.
On this scale the mass of magnesium is 24, since magnesium atoms are twice as "heavy" as carbon atoms. So we would say the relative atomic mass of magnesium is 24. This means that magnesium atoms have twice the mass of carbon atoms. The relative atomic mass of magnesium is therefore 24; we use the symbol Ar for relative atomic mass. So if we weigh out 24g of magnesium we will have the same number of atoms as we have in 12g of 12C; that is 1 mole of magnesium atoms. The only difference is the magnesium atoms are twice as massive as carbon atoms.

Similarly carbon atoms are 12 times as "heavy" as hydrogen atoms, so it follows that 12g of carbon and 1g of hydrogen contain the same number of atoms, that is 1 mole of atoms. The relative atomic mass expressed in grams for all elements will contain 1 mole of atoms.
 1 mole of carbon or 12 grams of carbon is
quite a large pile of carbon and obviously atoms are very very small, so there is going to be
quite a large number of atoms in that pile of carbon. In fact it's an unbelievable huge
number of carbon atoms, there are: 600 000 000 000 000 000 000 000 atoms of carbon in the
charcoal block opposite! That is 6.022 x 1023 atoms. That's a lot! This number is often called Avogadro's number;
after the Italian scientist Amedeo Avogadro. Imagine having to
count them! Well that is why we don't, we weigh them. That is the beauty of the mole. By simply
weighing out 12g of carbon we know exactly how many atoms we have. If you weigh out 1.2g of carbon;
that is 1/10th of a mole or 0.1 moles then you can easily work out how many atoms of carbon you have:
1 mole of carbon or 12 grams of carbon is
quite a large pile of carbon and obviously atoms are very very small, so there is going to be
quite a large number of atoms in that pile of carbon. In fact it's an unbelievable huge
number of carbon atoms, there are: 600 000 000 000 000 000 000 000 atoms of carbon in the
charcoal block opposite! That is 6.022 x 1023 atoms. That's a lot! This number is often called Avogadro's number;
after the Italian scientist Amedeo Avogadro. Imagine having to
count them! Well that is why we don't, we weigh them. That is the beauty of the mole. By simply
weighing out 12g of carbon we know exactly how many atoms we have. If you weigh out 1.2g of carbon;
that is 1/10th of a mole or 0.1 moles then you can easily work out how many atoms of carbon you have:
 It follows then that:
It follows then that: