

Alcohols all contain the hydroxyl functional group (R-OH), it is the
presence of this -OH hydroxyl group which is responsible for the
reactions and much of chemistry of alcohols.
Alcohols are named from the corresponding alkane by replacing the -e at
the end of the alkane name by -ol. Alcohols
can be classified as primary, secondary or tertiary depending on the number of
alkyl groups attached to the carbon atom bonded to the hydroxyl group; for example:
| primary alcohols | secondary alcohols | tertiary alcohols |
|---|---|---|
| contain 2 hydrogen atoms and one alkyl group (R) attached to the carbon bonded to the -OH functional group | contain 1 hydrogen atom and two alkyl groups (R) attached to the carbon bonded to the -OH functional group | contain three alkyl groups (R) attached to the carbon bonded to the -OH functional group |
This difference in structure between primary, secondary and tertiary alcohols is shown in the image below:

Many of the reactions of alcohols are the same, since they they all contain the same hydroxyl functional group (R-OH). However one area where the reactions of primary, secondary and tertiary alcohols is different is in their reactions with oxidising agents such as acidified potassium dichromate.

One thing that is often confusing in chemistry is the use of the words oxidation and reduction; this is simply because there are so many different definitions that chemists use for these two words, for example oxidation can be described as:
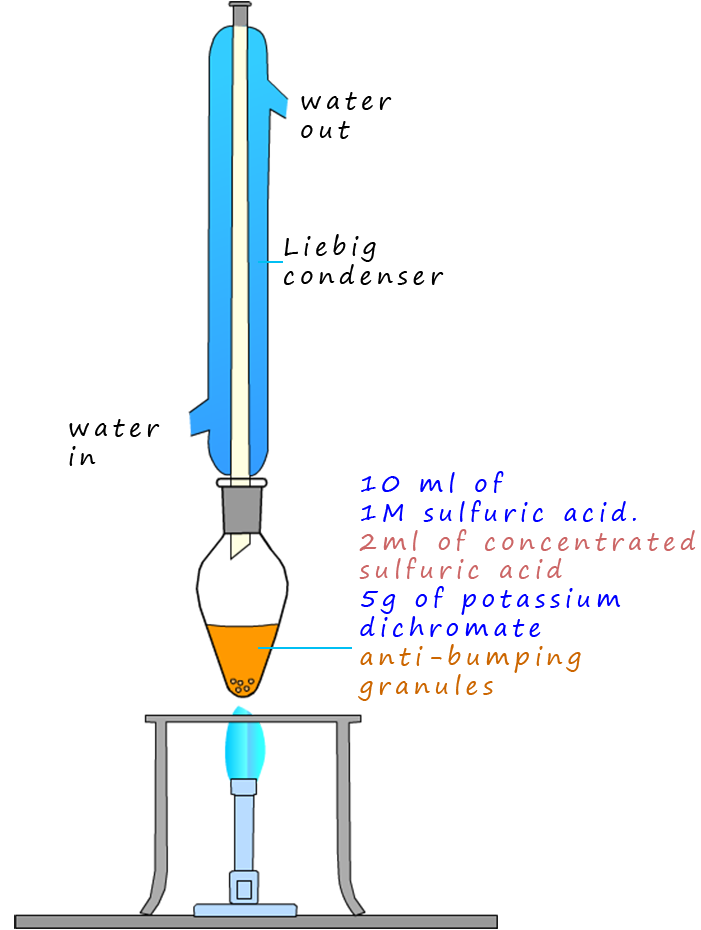
As an example of the oxidation process consider the oxidation of the primary alcohol ethanol to the aldehyde ethanal, the apparatus set-up to carry out this oxidation reaction is shown below. The set-up is simple distillation; the alcohol ethanol has a boiling point of 780C while the aldehyde ethanal has a boiling point of only 230C. The oxidising agent; that is the potassium or sodium dichromate and sulfuric acid are added to the pear shaped flask first then the alcohol ethanol is dripped in slowly. Gently warming will cause the ethanal vapour to enter the Liebig condenser where it will liquefy and collect in the flask. It is good practice to cool the flask in iced water since the boiling point of the ethanal is close to room temperature (250C) and from my experience ethanal vapour is very good at giving you a thumping headache!

Equation for this oxidation reaction are often written as shown below, here the symbol [O] is used to represent the oxidising agent.
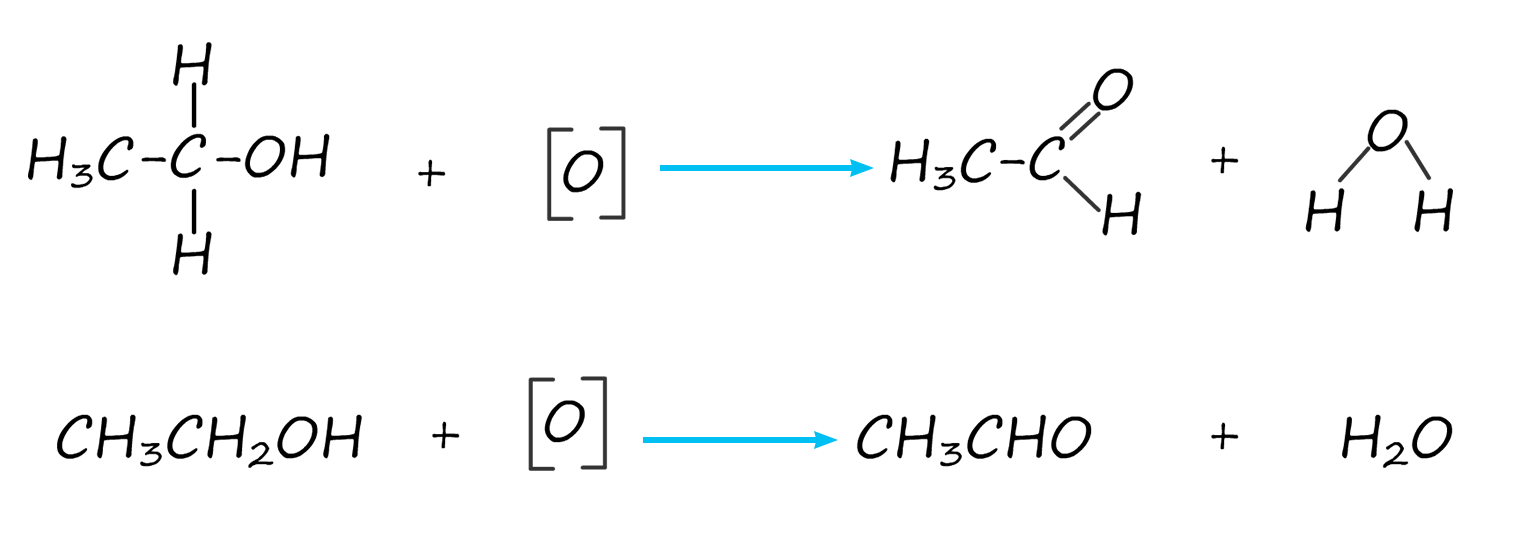
The aldehyde ethanal produced by the oxidation
of the alcohol ethanol can be further oxidised
to a carboxylic acid, in this case ethanoic acid. The same acidified potassium
or sodium dichromate can be used to oxidise
the ethanal but this time the conditions are made more severe. Concentrated
sulfuric acid is used along with more dichromate in order to carry out this
second oxidation reaction.
This time we will define oxidation as the addition of oxygen, slightly
different from the example above where we defined oxidation as a loss
of hydrogen, but remember at the end of the day it all amounts to exactly to same thing! Ethanal can be
oxidised to
ethanoic acid. However the apparatus set-up will have to be modified.
The ethanol as above is oxidised to
the aldehyde ethanal, however ethanal is very volatile and evaporates easily, so
a reflux experiment will have to be set-up.
The set-up is shown opposite. Here the alcohol ethanol is oxidised to aldehyde ethanal which
being volatile will evaporate and enter the Liebig condenser where
it will be liquefied and simply drip back down into the oxidising mixture
of sulfuric acid and dichromate. After around 20
minutes or so the oxidation reaction should be complete. Simply rearrange
the apparatus back into a distillation set-up and distil off
the ethanoic acid (boiling point 1180C) produced; equations to show this further oxidation of the aldehyde ethanal to the carboxylic acid ethanoic acid are shown below:
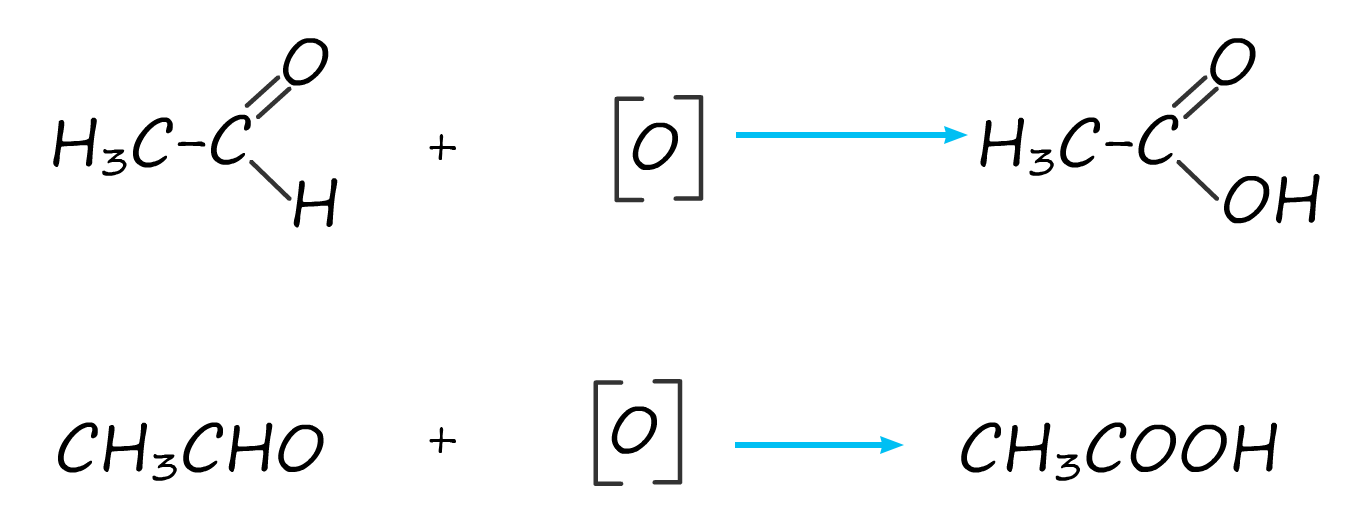
Secondary alcohols as mentioned above are oxidised to ketones and since ketones have no hydrogen atoms attached to the carbonyl carbon they cannot be oxidised further using acidified dichromate as the oxidising agent. The equation below show the oxidation of the secondary alcohol propan-2-ol to the ketone propanone.

The dichromate ion (Cr2O72-) is a bright orange colour. It contains chromium atoms in the +6 oxidation state; it is the presence of these ions which are responsible for the orange colour of the dichromate ion. When acidified dichromate solution is mixed with a primary or secondary alcohol the Cr+6 ion is reduced to the green Cr3+ ion. This is shown in the image below. However as mentioned earlier tertiary alcohols cannot be oxidised with acidified dichromate and when a warm acidified dichromate solution has a tertiary alcohol added no colour change is observed. This is outlined in the image below:

Oxidising alcohols with
acidified potassium dichromate will enable you to distinguish between
tertiary alcohols and primary/secondary
alcohols. However it cannot distinguish between primary and secondary
alcohols, as both of these react with the
acidified dichromate.
However it is possible to test the products of the oxidation of primary and
secondary alcohols which will enable us to differentiate
between them.
Aldehydes are readily oxidised to
carboxylic acids but ketones, the oxidation
product of secondary alcohols are not readily
oxidised, unless powerful oxidising agents are used. So by using mild
oxidising agents such as Tollens' reagent or Fehling's solution
it is possible to differentiate between aldehydes and
ketones and hence primary alcohols from secondary
alcohols.
Fehling's solution is a beautiful dark blue coloured solution,
it made by mixing copper(II) sulfate solution and a solution
of sodium tartrate in potassium or sodium hydroxide. If you were to simply add an
alkaline solution such as sodium or potassium
hydroxide to a solution containing a transition metal ion (M2+) such as Cu2+ you will simply produce a solid precipitate of the metal hydroxide, in this case
copper hydroxide. However the tartrate ions form a complex ion with the Cu2+ ion which prevents
this precipitate of
copper hydroxide forming.
If about 2ml of an aldehyde is mixed with a few mls of
Fehling's solution in test-tube and then placed in a hot water bath the aldehyde
will reduce the Cu2+
ions present in the Fehling's solution to form the Cu+ ion, which in the alkaline conditions forms a orange-brown
precipitate of copper (I) oxide.
We can show this as:
The oxidation half-equation shown below outlines how the aldehyde is oxidise to the carboxylic acid, however since the Fehling's solution is alkaline the salt of the carboxylic acid will be produced instead of the carboxylic acid.
Combining the two half-equations gives the overall redox equation:
Tollens' solution is prepared by adding about 5ml of silver nitrate solution into a boiling tube then adding a drop of sodium hydroxide solution. This will produce a brown precipitate of silver(I)oxide, next add drop by drop aqueous ammonia solution until the precipitate of silver oxide dissolves. This new solution is called Tollens' solution and it contains the silver diammine complex [Ag(NH2)]+OH- or simply silver ions (Ag+). The equations below summarise how the Tollens' solution is prepared.
Addition of ammonia drop by drop now forms the Tollens' solution.
If a few drops of aldehyde are then added to the Tollens' solution and warmed on a water bath the aldehyde reduces the silver ions (Ag+) present in the Tollens' reagent to metallic silver. This is usually seen as a silver coating on the walls of the test-tube, it is often called a silver mirror. This is shown below:

Equations for this redox reaction are shown below:
Or we can write the reduction half-equation using the diammine complex:
The half-equation for the oxidation of the aldehyde (RCHO) can be written as shown below. Note that the Tollens' solution is alkaline; therefore the salt of the carboxylic acid will be produced instead of the carboxylic acid. Review the work you did on writing half-equations if you need help in writing these equations.
The oxidation reaction produces 2e while the reduction half-equation only requires 1e. So to balance these equations and get an overall equation the reduction half-equation needs to be multiplied by x2.
The diagram below summaries the results of the Fehling's and Tollens' tests on aldehydes. Ketones do not react with Fehling's or Tollens' solutions.
