

This page follows on from the pages on an introduction to shapes of molecules and using VSEPR theory to find the shapes of molecules with no lone pairs. This page will focus on how to find lone pairs of electrons in tetrahedral molecules and the effects these lone pairs of electrons have on the shape of a molecule.
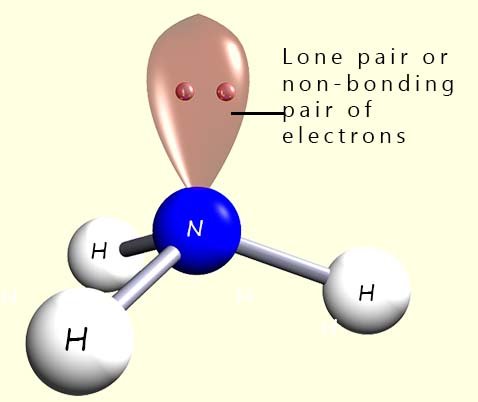 However a tetrahedral molecule requires 4 atoms or groups around the
central atom but in the example above using ammonia there are only 3 hydrogen
atoms around the central nitrogen atom not 4. Ammonia contains 3 hydrogen atoms which will account for 6 electrons in three covalent bonds in the outer valency shell and a
lone pair or
a non-bonding pair of electrons; which gives a total of 8 electrons in the valency shell. When deciding on the final shape of a
molecule it is vital that you locate any of
these lone pairs.
However a tetrahedral molecule requires 4 atoms or groups around the
central atom but in the example above using ammonia there are only 3 hydrogen
atoms around the central nitrogen atom not 4. Ammonia contains 3 hydrogen atoms which will account for 6 electrons in three covalent bonds in the outer valency shell and a
lone pair or
a non-bonding pair of electrons; which gives a total of 8 electrons in the valency shell. When deciding on the final shape of a
molecule it is vital that you locate any of
these lone pairs.
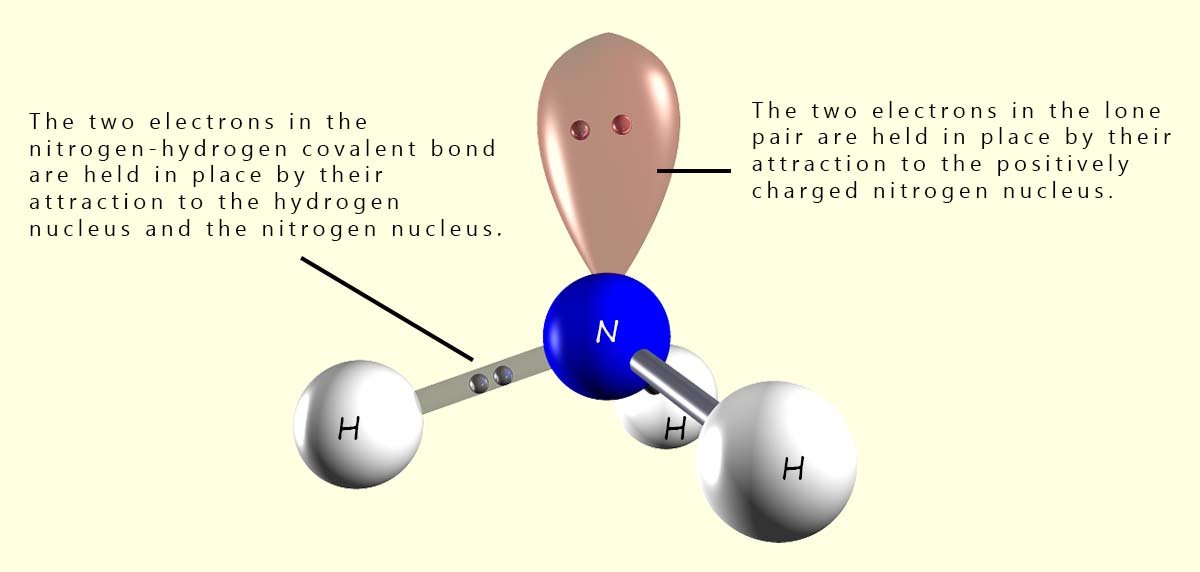
In a normal covalent bond between two atoms each atom contributes one electron to the covalent bond and these two electrons are held in place by their attraction to the two positively charged nuclei of the bonding atoms. However lone pairs or non-bonding pairs of electrons are slightly different. Here we have 2 electrons in the lone pair but they are being held in place by the attraction of only one nucleus. This means that the electrons in a lone pair are not held as tightly as those in a bonding pair of electrons and as a consequence of this lone pairs take up more space than regular bonding pairs of electrons. This is outlined in the diagram below.
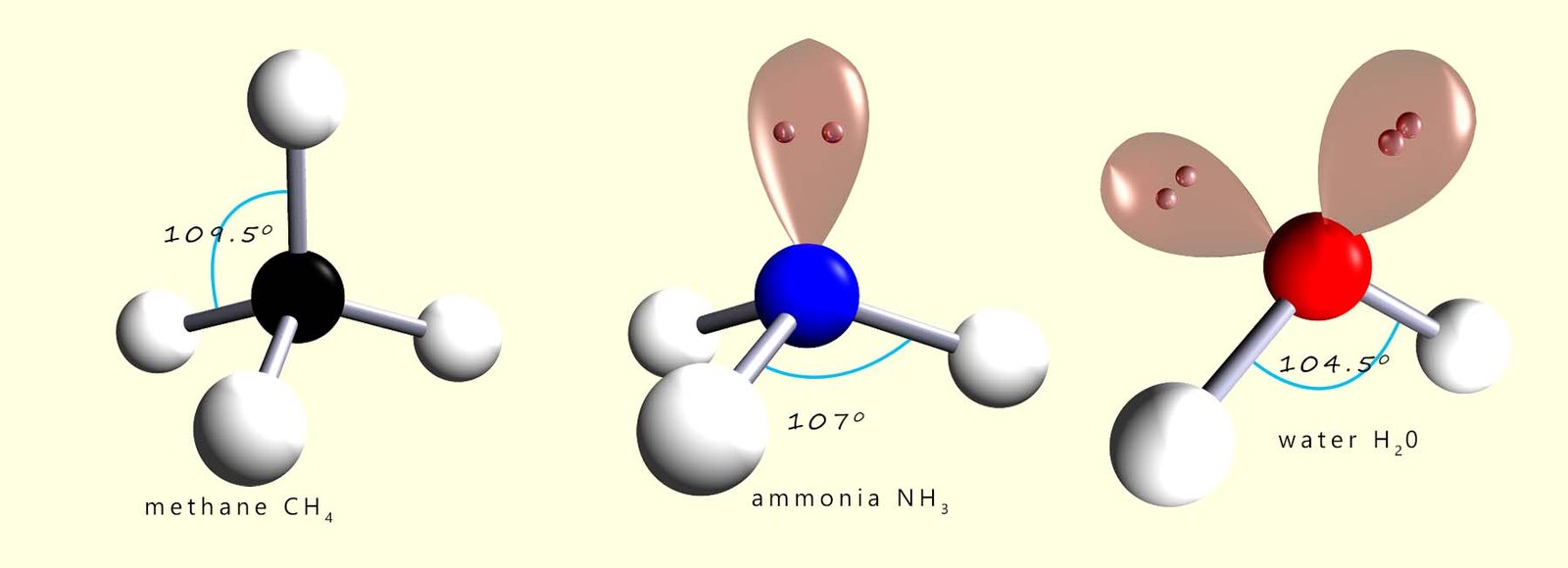
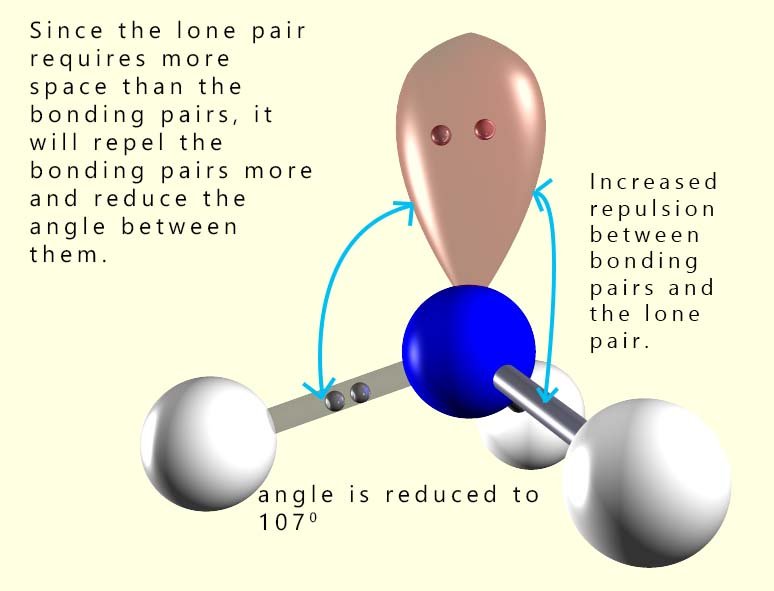 In a normal tetrahedral molecule with no
lone pairs and 4 bonding pairs of electrons all the bond angles would
be 109.5°. However since the lone pair takes up more space than a bonding pair of electrons it will compress or
repel the other three bonding pairs of electrons and reduce the bond angle between them to below 109.5°. The single lone pair
will force the 3 bonding pairs closer together and the new bond angle between them will be 107°.
In a normal tetrahedral molecule with no
lone pairs and 4 bonding pairs of electrons all the bond angles would
be 109.5°. However since the lone pair takes up more space than a bonding pair of electrons it will compress or
repel the other three bonding pairs of electrons and reduce the bond angle between them to below 109.5°. The single lone pair
will force the 3 bonding pairs closer together and the new bond angle between them will be 107°.
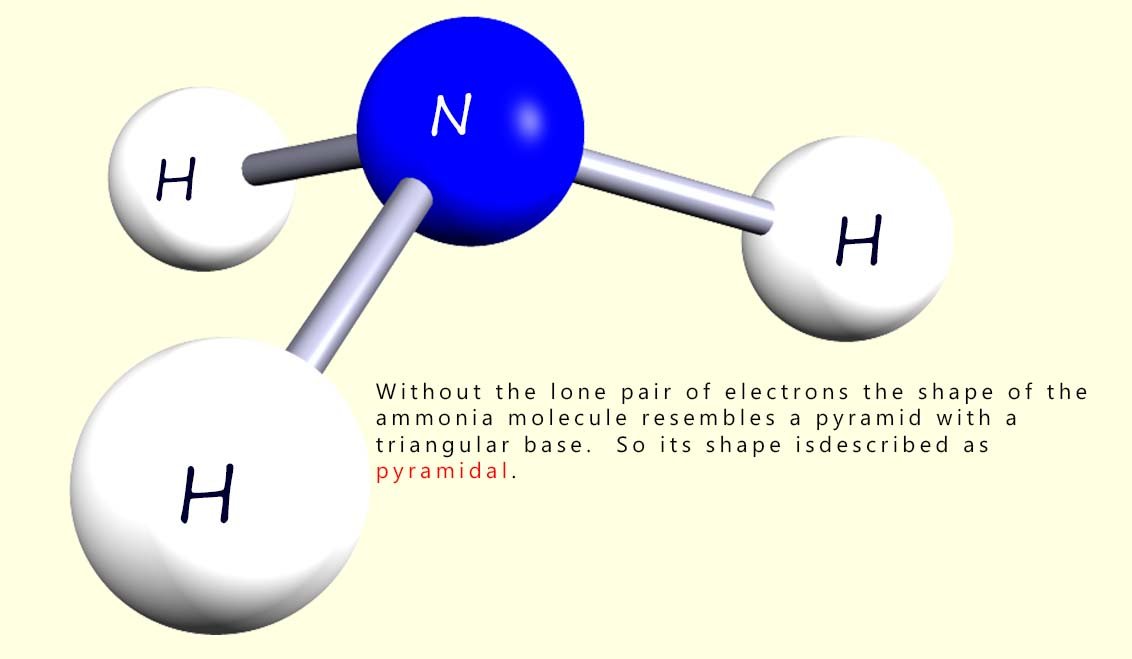
In deciding on the shape of the molecules you need to be aware of the presence of any lone pairs of electrons but they are not taken into account when deciding on the overall shape of the molecule. So what shape is the ammonia molecule then? Well the image below shows the ammonia molecule without its lone pair of electrons. The shape is no longer tetrahedral as this requires 4 atoms around the central atom. If you look at the molecule it resembles a pyramid with triangular sides. So its shape is described as trigonal pyramidal or simply pyramidal.
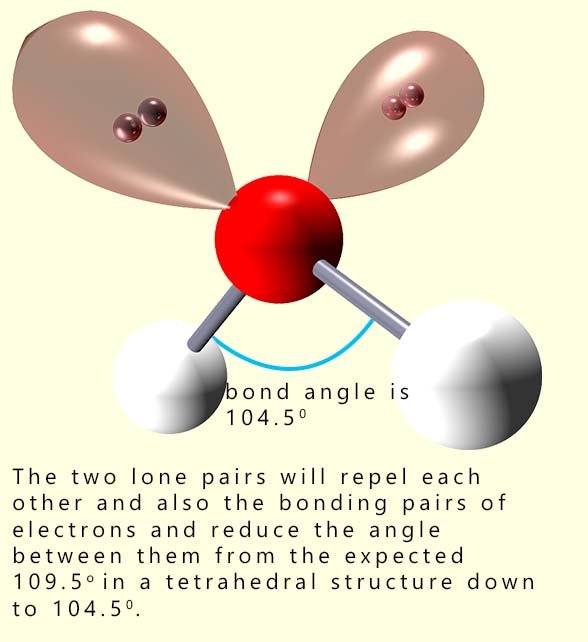 As another example of a small molecule with lone pairs consider a molecule of water (H2O). What would be the
shape of a molecule of water? Well as before to work out the shape of this molecule simply use the VSEPR rules:
As another example of a small molecule with lone pairs consider a molecule of water (H2O). What would be the
shape of a molecule of water? Well as before to work out the shape of this molecule simply use the VSEPR rules:
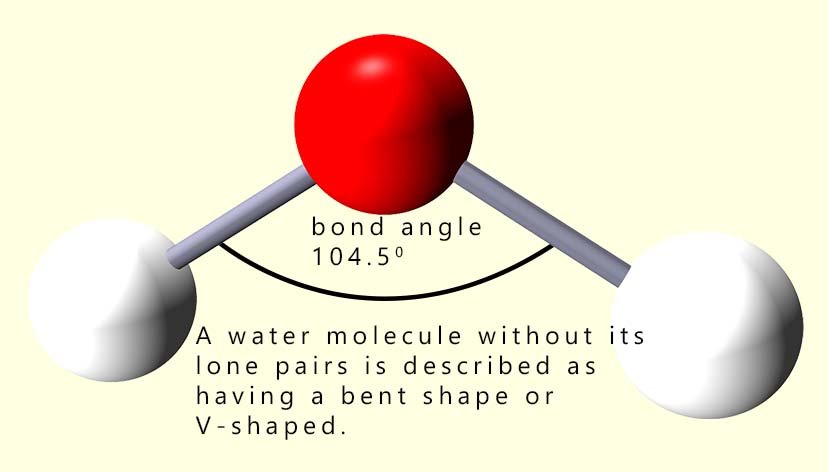
Remember that we do not consider the lone pairs when deciding on the final shape of the molecule. So try to imagine a water molecule without its lone pairs of electrons. Without the presence of the lone pairs the water molecule is described as having a V-shape or bent shape. This is shown below:
We mentioned earlier that lone pairs of electrons require more space than bonding pairs. In the ammonia molecule there is a single lone pair of electrons and this compressed or squashed down the bond angles between the bonding pairs by over 2° from 109.5 to 107°. Water has 2 lone pairs of electrons and this means that the bonding pairs are going to be compressed or forced even closer together. In the diagram above you can see that the bond angle between the hydrogen atoms and the oxygen atom is squashed down from 109.5° to 104.5°
Complete the quick activity below by dragging the sliders to identify the bond angles in ammonia and water molecules.
Set the bond angle for the ammonia and water molecules, then press “Check”.