

The table below contains the first six alkanes; the
alkanes are a family of saturated
hydrocarbons
that you met in gcse science. Now many of the molecules you will meet in your A-level organic chemistry course
are in some way based on this family of molecules, simply because many of the organic compounds you will meet in chemistry are made
by replacing one or more of the hydrogen atoms in an alkane
molecule with another atom
or a group of atoms. These new atoms or group of atoms maybe responsible for the reactions of the
molecule, we call these reactive parts of a molecule the functional group and you will very quickly realise that these functional groups are the key to understanding many of the reactions that molecules undergo.
The alkanes are a family of saturated hydrocarbons that contain the C-C and C-H groups only, that is they contain a single covalent bond between all the atoms in the molecule. The general formula for the alkanes is CnH2n+2, now the table below is included as a simple reminder of the names, structure and formula for the first six alkanes. I included the 3d-structures to remind you of the fact that there is free rotation around the C-C and C-H bonds and that the geometry around all the carbon atoms in the alkane is tetrahedral.
| Name | Number of carbon atoms | Molecular Formula | Structure |
|---|---|---|---|
| methane | 1 | CH4 | 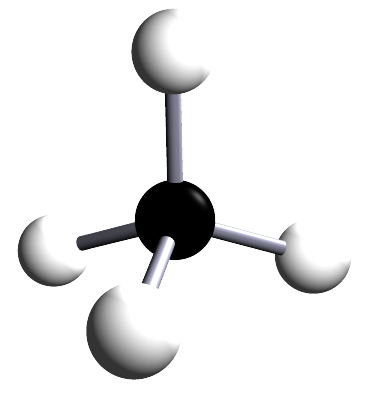 |
| ethane | 2 | C2H6 | 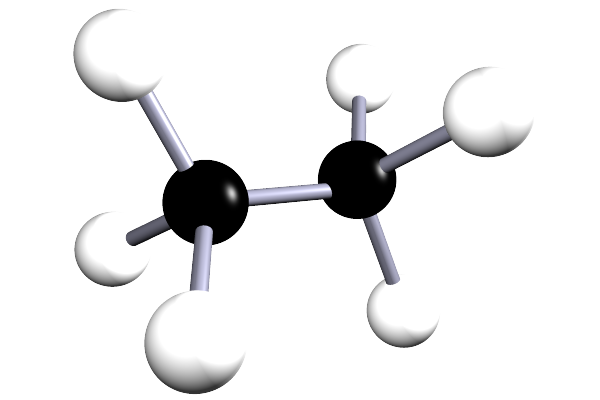 |
| propane | 3 | C3H8 | 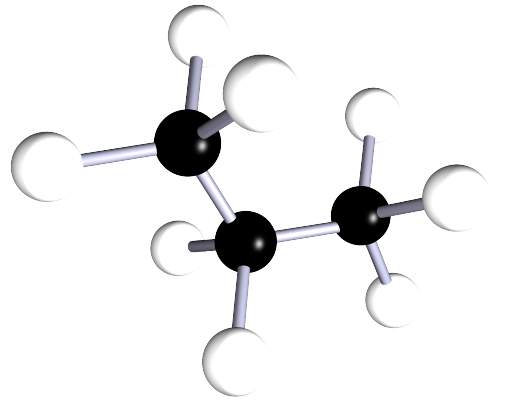 |
| butane | 4 | C4H10 | 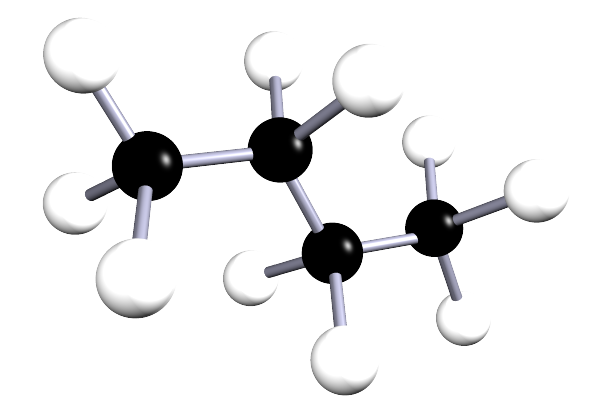 |
| pentane | 5 | C5H12 | 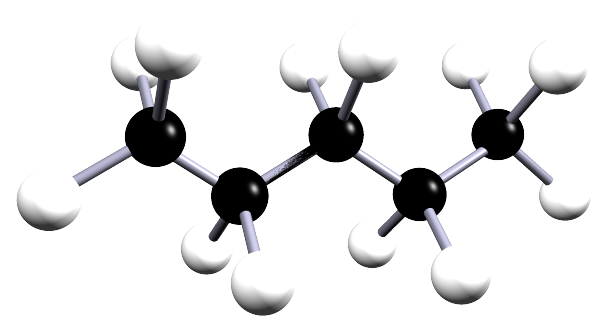 |
| hexane | 6 | C6H14 | 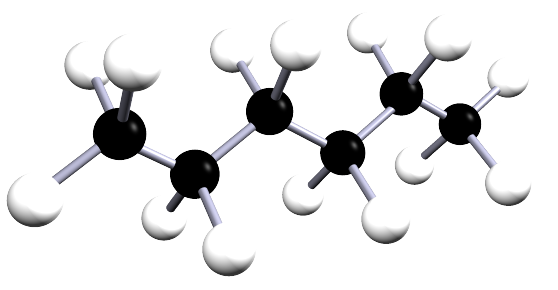 |

Hexane (shown opposite) is a saturated alkane with the molecular formula C6H14. If you had a model kit with 6 black atoms of carbon
and 14 white atoms of hydrogen I am sure you could build lots of different shaped molecules
other than the one shown. In fact you could build five different shaped molecules
using all the carbon and hydrogen atoms. These molecules would all
have the same molecular formula
but they will all look different from each; for example the image below shows two molecules, both of which have the same molecular formula (C6H14 but they have different structures, they obviously look different from each other.

Molecules such as this which have the same molecular formula but different structures are called structural isomers.
A structural isomer is a molecule with same molecular formula but a different structural formula
There are 3 types of structural isomers that you need to know about, these are:
How would we go about naming the two chain isomer of hexane shown above? To name the compounds we first have to identify the longest chain of carbon atoms present in each molecule. The number of carbon atoms present in the longest chain will tell us the root name for the compound. This is outlined below:
| Number of carbon atoms present in the longest carbon chain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| root name | meth | eth | prop | but | pent | hex | hept | oct | non | dec |
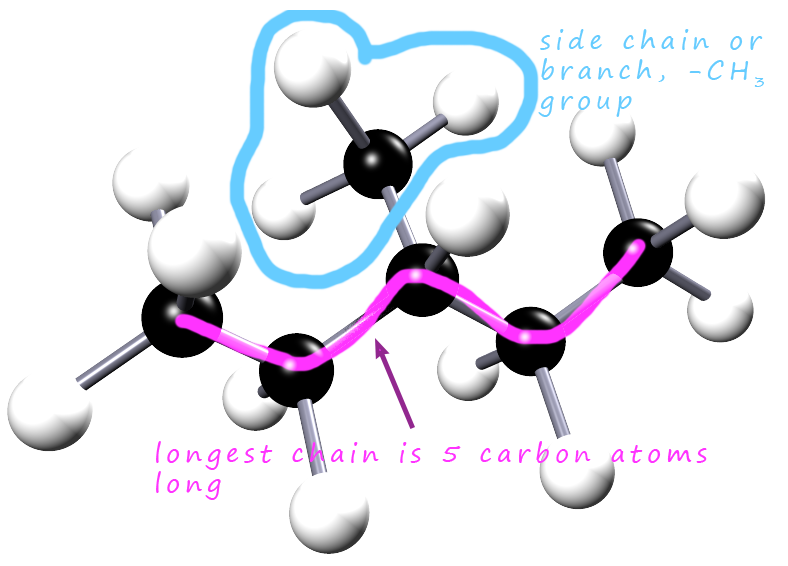
In the example above hexane clearly has 6 carbon atoms; so the root name will
be hex-. The second part of the name -ane; tells us
that it belongs to the alkane family. Recall from gcse
science that the alkanes are all saturated hydrocarbons, that is they contain only single covalent bonds. In
the second example; which is also shown opposite the longest chain
of carbon atoms is only 5 atoms long and there is a side chain or branch on the third carbon atom. This branch contains
the -CH3 group. These branches
are very common in molecules and are named simply from the number of
carbon atoms present in them as shown in the table below.
So to name our isomer of hexane we have:
| Number of carbon atoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| branch name | methyl | ethyl | propyl | butyl | pentyl | hexyl | heptyl | octyl | nonyl | decyl |
| formula | CH3- | C2H5- | C3H7- | C4H9- | C5H11- | C6H13- | C7H15- | C8H17- | C9H19- | C10H21- |

So to name our isomer we have:
If we had started numbering the carbon atoms from the other end of the molecule then we would have:

The -CH3 branch is on carbon atom number 4. The
longest chain of carbon atoms is still 6 carbon
atoms long, so the name of the molecule this time would be 4-methylhexane.
This example demonstrates an important rule when naming compounds; that
is that any substituents or branch chains must be given the lowest
number possible. So the molecule above would be named 3-methylhexane and NOT 4-methylhexane.
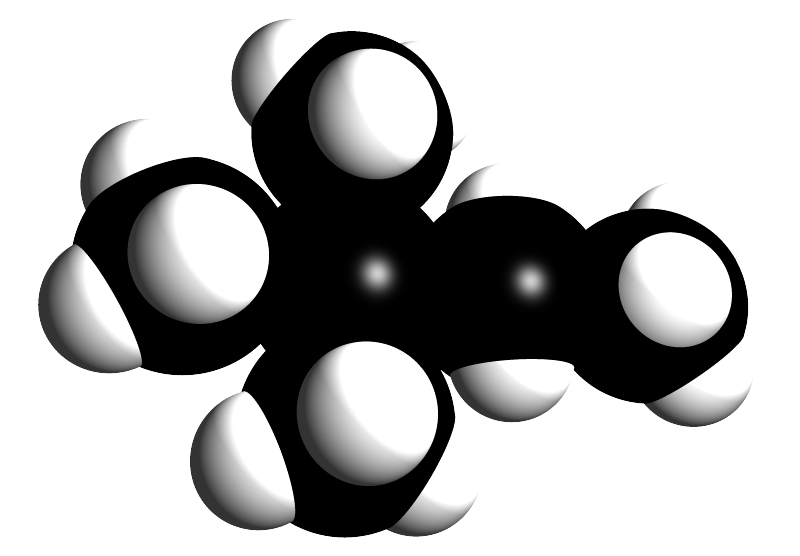
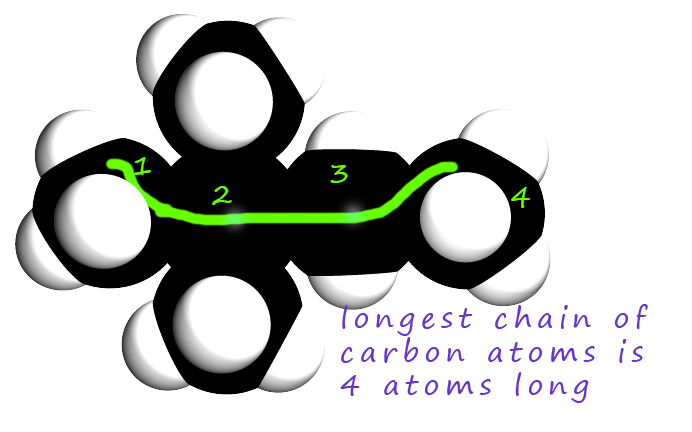
The image opposite shows a space filled model of a branched chain hydrocarbon molecule. What is the systematic name of this molecule?
Use the same method as shown above:
Hexane as mentioned above has five in the structural isomers; specifically it has five chain isomers. Let's have a quick look at the formulae for these isomers.
The image below shows four of the five chain isomers of hexane:
