

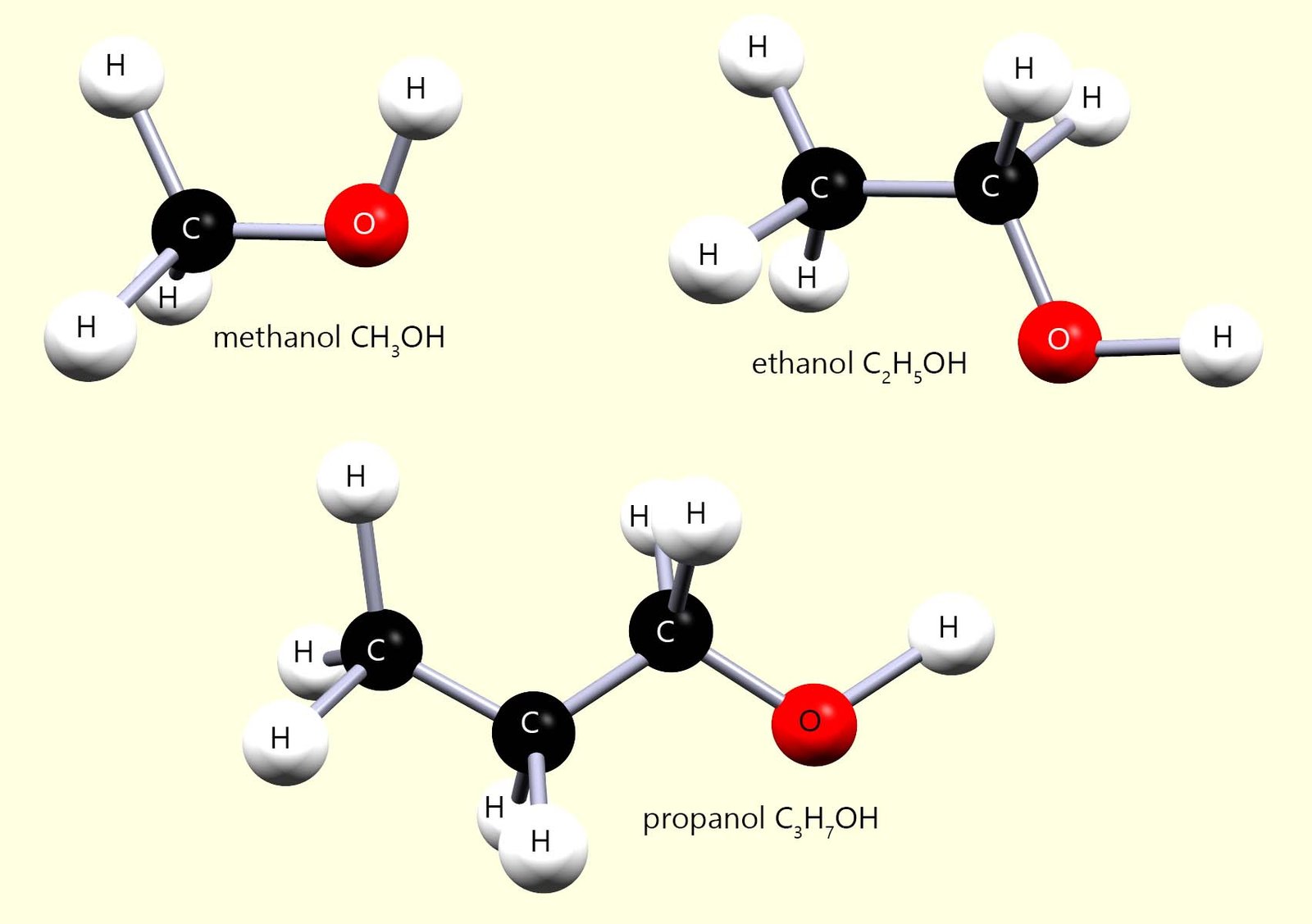
The word alcohol is perhaps most commonly associated with alcoholic drinks, unfortunately most alcohols are toxic substances and are definitely not suitable as drinks. You may recall a rather tragic story that headlined recently in Laos, South East Asia where several tourists consumed bootleg alcoholic shots, unfortunately these shots were laced with methanol and tragically this resulted in the death a six of these backpackers.
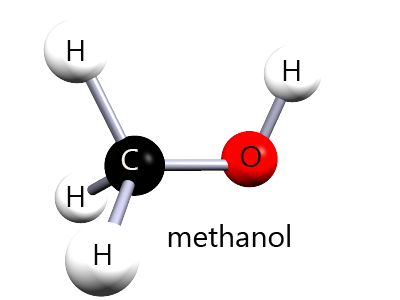
⚠️ Methanol and ethanol are both alcohols|, but methanol is highly toxic because it is oxidised in the body to methanoic acid.
🌍 In November 2024 a tragic methanol poisoning incident occurred in Vang Vieng, Laos, South East Asia when Australian, Danish, British and American tourists unknowingly drank alcoholic shots which were contaminated with methanol rather than ethanol. The drinks they consumed were believed to be unregulated or illegally produced bootleg alcohol.
🤕 Early symptoms of methanol poisoning such as headache, dizziness and nausea were mistaken for a severe hangover, delaying treatment. However it can take up to 24 hours for victims to start showing serious signs of illness which include: nausea, vomiting and abdominal pain which can escalate into hyperventilation and breathing problems.
💀 If not treated fatality rates for methanol poisoning are often reported to be as high as 20% to 40%.
⚰️ Even small amounts of methanol (around 30 ml) can be fatal, as methanoic acid causes severe metabolic acidosis (a condition where there is too much acid in the body's fluids) and damage to the optic nerve leading to blindness.
💀 In this case, six tourists later died in hospital. This example highlights how a small chemical difference between two alcohol molecules can have fatal consequences.
The one alcohol which is less harmful is ethanol (C2H5OH) , although it still causes many people to do very silly things when taken in excess! Ethanol (C2H5OH) has been made for thousands of years as an alcoholic drink in a process called fermentation.


Alcohols are an important group of organic compounds that have a wide range of uses including:

In A-level chemistry the most important alcohol you need to consider is ethanol, now there are two main methods used to produce the alcohol ethanol.
In this topic you need to understand and be able to describe the conditions, advantages and disadvantages of each of the two methods described above to produce ethanol and be able to compare them in terms of rate, yield, cost and environmental impact. Other laboratory methods for making alcohols are covered later in the course and are not used for the large-scale manufacture of ethanol.
The fermentation process is slow and can take days or even weeks. Fermentation is a natural process; it is a form of anaerobic respiration. An equation for fermentation is:
The method outlined above to produce ethanol is a traditional batch process which, while requiring fairly inexpensive equipment and machinery but is labour intensive which can make it relatively expensive overall.
The ethanol produced by fermentation can also be used as a biofuel . When ethanol is produced from plant material and is then used as a fuel, it is known as bioethanol. Biofuels are a renewable source of energy since they are produced from plant-based materials 🌱. For example sugarcane in Brazil and corn in the USA can be grown and then fermented to form ethanol, which is used as bioethanol for cars. Bioethanol is often described as being carbon neutral because the amount of carbon dioxide released into the atmosphere when it is burned together with the carbon dioxide released during fermentation should be equal to the amount of carbon dioxide removed from the atmosphere during photosynthesis in the plant. This is outlined in more detail in the table below:
♻️ Carbon balance of bioethanol production and use
| 🌱 Removal of carbon dioxide from the atmosphere | 🏭 Addition of carbon dioxide to the atmosphere |
|---|---|
|
🌿 Photosynthesis 6CO2 + 6H2O → C6H12O6 + 6O2 Here 6 moles of CO2 are removed for every mole of glucose formed. |
🧪 Fermentation C6H12O6 → 2C2H5OH + 2CO2 Here 2 moles of CO2 are released for every mole of glucose fermented. 🔥 Combustion 2C2H5OH + 6O2 → 4CO2 + 6H2O Here 4 moles of CO2 are released for every 2 moles of ethanol burned. |
| ✅ Total CO2 removed = 6 moles | ⚠️ Total CO2 released = 6 moles |
By studying the information provided in the table above it might appear at first glance that the use of biofuels is indeed a carbon neutral process. However this may not be the whole story because you also need to consider the "hidden carbon dioxide" released during the production and use of these biofuels.

The information in the table above might suggest that using ethanol as a biofuel is a carbon neutral process.
However, the table can be misleading because it does not include the extra CO2 released by processes such as growing,
harvesting, transporting and processing the crops and producing the ethanol by fermentation.
Similarly if the ethanol is used as a biofuel it needs further processing and transport to where it is used, which also releases additional
CO2 into the atmosphere.
There is also increasing concern about the land🌾 used to produce biofuels. Land used to grow biofuel crops may replace land that could grow food 🌾 and in some cases habitats (including tropical rainforest) are cleared to make way for crops. With a growing world population and increasing demand for food, many people argue it is unethical to grow crops to fuel cars when millions of people do not have enough to eat.
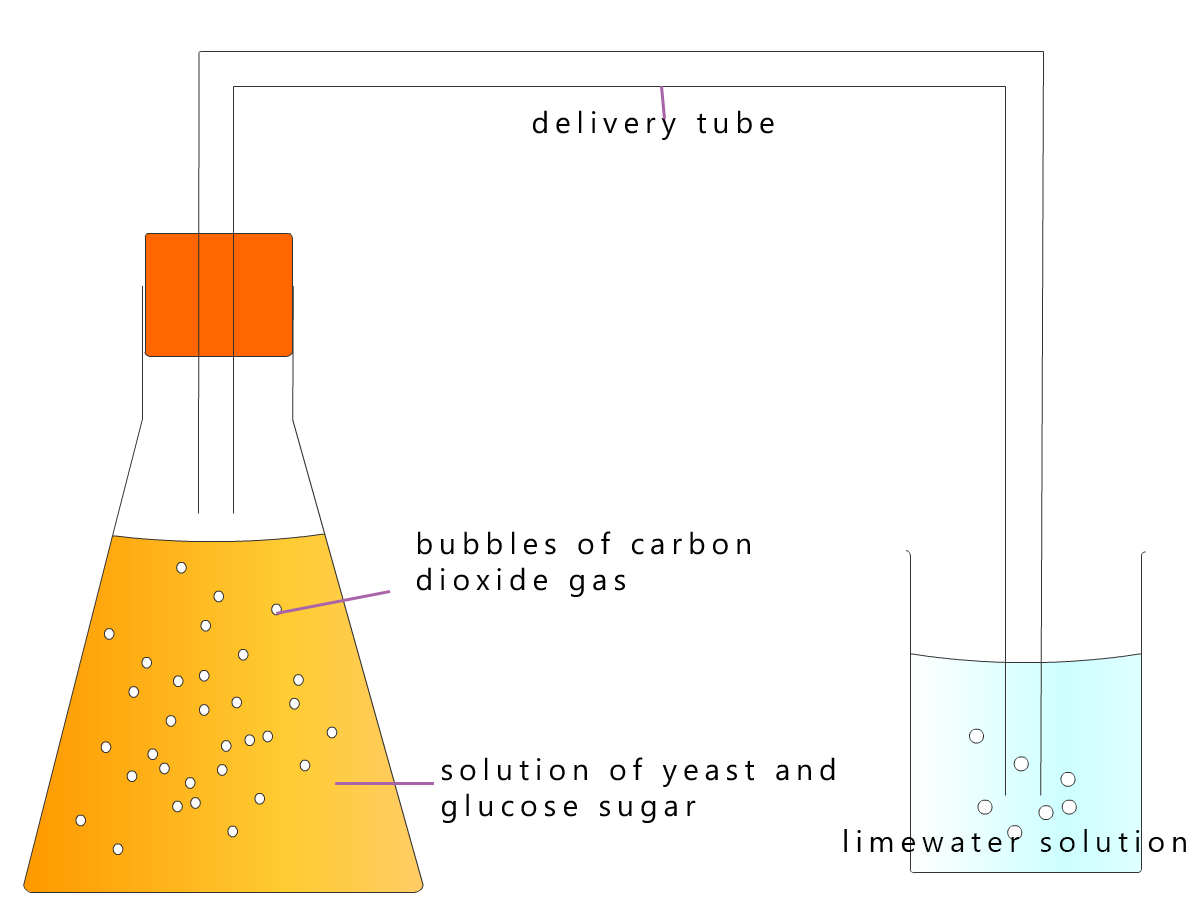
Making alcohol using yeast in the lab is very simple. All you need is a source of sugar (for example, sweet fruit such as apples, pears, strawberries, blackberries or grapes). The fruit is squashed, added to lukewarm water and placed in a container with a lid or air lock somewhere warm, like an airing cupboard or beside a radiator. Once the yeast is added to this sugary solution then fermentation will begin and the sugars from the fruit will start to change into the alcohol ethanol.
After about 2 weeks filter the mixture to remove the yeast and any other solids present. That is it.... easy, you have made ethanol!
Yeast is a fungus (a living organism) that produces
enzymes called
zymase which convert the
sugar glucose into
ethanol and
carbon dioxide.
This process is a form of anaerobic respiration meaning respiration without
oxygen present.
The enzymes work best at an
optimum temperature of around
30–40°C.
If the fermenting mixture gets
too hot the
enzymes become
denatured
and fermentation stops.
If the mixture is kept too
cold the
enzymes work much more slowly so
fermentation is slow.
Air must be excluded because
oxygen (and bacteria in the air) can lead to
oxidation of
ethanol to
ethanoic acid.
One of the main uses for alcohol is as a sterilising agent to kill bacteria and fungi. For example hand sanitisers often contain a high percentage of ethanol which kills many microbes. This matters in fermentation too since once the concentration of ethanol in the fermenting mixture reaches around 10–15% fermentation slows down and can stop because ethanol inhibits and eventually kills the yeast. To produce alcoholic spirits such as whisky or vodka which may contain around 40% alcohol the alcoholic mixture from fermentation needs to be distilled.
Alcohol (ethanol) used in alcoholic drinks such as beer, wine and spirits are made by fermentation. However, ethanol can also be made by a method called direct hydration. This method produces ethanol quickly. It is a continuous process (whereas fermentation is a batch process) and in principle the only product is ethanol, so there is minimal separation problems compared with fermentation mixtures. However, it is a non-renewable process because the ethene feedstock comes from crude oil.
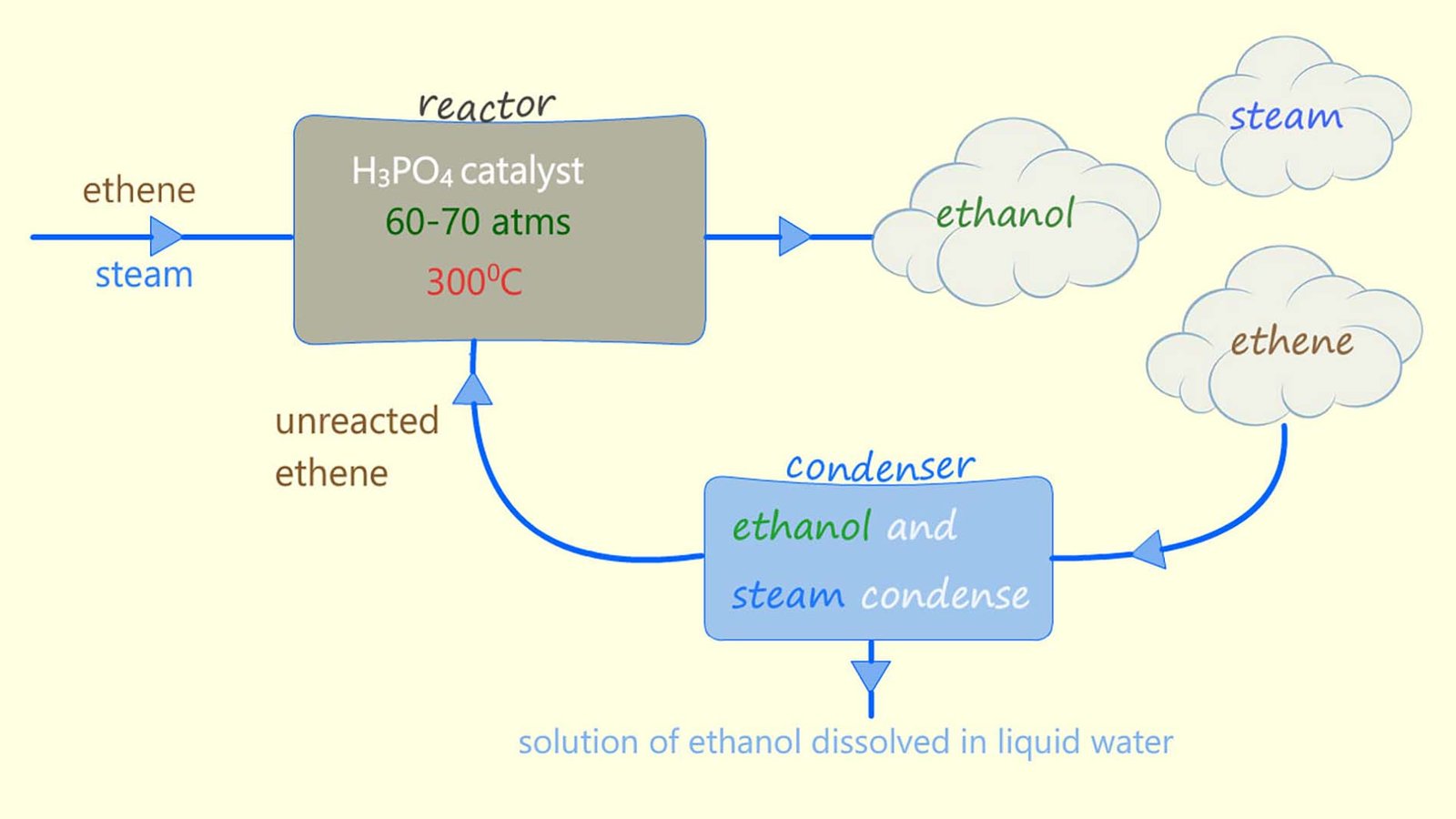
Alcohol used for industrial purposes such as solvents and in sanitisers is usually made by the direct hydration method rather than fermentation. Direct hydration involves adding steam across the C=C bond in an alkene molecule, usually ethene. The reaction is shown below. A catalyst (phosphoric acid), a high temperature (around 300°C) and a high pressure (about 65 atmospheres) are needed to get this reaction to occur smoothly.
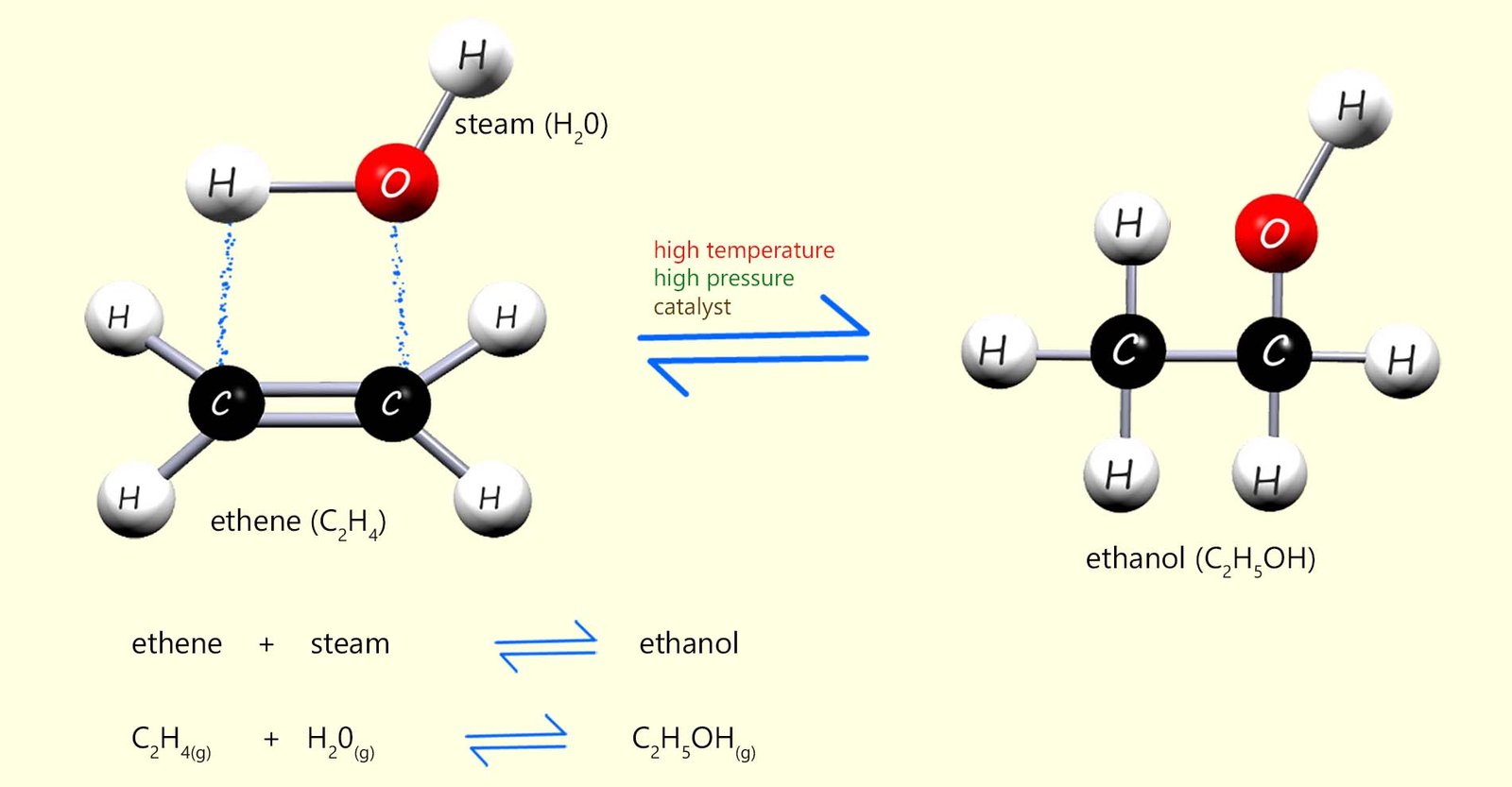
Equations for this reaction are:
From the equation in the image above you can see that this hydration reaction is a reversible reaction. This means that the products of the reaction will be a mixture of ethanol, steam and unreacted ethene, in fact very little of the ethene and steam react first time when they first enter the reaction chamber. The reaction mixture of substances formed from the hydration reaction leaves the reaction vessel and enters a condenser where the steam and ethanol formed are removed and the unreacted ethene is recycled back through the reaction vessel. This is outlined below:
| 🧪 Method | 🌱 Fermentation | 🏭 Direct hydration |
|---|---|---|
| Type of process | Biological (uses enzymes from yeast) | Industrial chemical process |
| Raw materials | Glucose (from plant material) | Ethene + steam |
| Conditions | Warm (30–40 °C), anaerobic | High temperature, high pressure, catalyst |
| Rate of reaction | Slow ⏳ (days or weeks) | Fast ⚡ (continuous process) |
| Purity of ethanol | Dilute ethanol mixed with water | High-purity ethanol |
| Renewable? | Yes 🌱 (plant-based) | No ⛽ (ethene from crude oil) |
| Main uses | Alcoholic drinks, bioethanol fuel | Industrial ethanol (solvents, sanitisers) |
Click the button below to try the quick 1 minute quiz on fermentation and direct hydration.
Click the correct method for each statement. Instant feedback, no stress 🙂
1) Uses enzymes produced by yeast
2) Needs a catalyst and high pressure
3) Produces ethanol mixed with water so it often needs distillation
4) Uses ethene as the starting material
5) Works best under anaerobic conditions
6) Is a continuous industrial process
7) Uses glucose (sugar) as the starting material
8) The reaction is reversible so unreacted gases are often recycled
Try the questions below; they are designed to help you avoid common mistakes that students make in their exams.
Decide if each statement is true or false. Then read the reason.
1) Fermentation produces pure ethanol.
2) Fermentation needs oxygen to work properly.
3) Direct hydration is a reversible reaction so unreacted ethene can be recycled.
4) Direct hydration is renewable because it uses steam.
5) Bioethanol is ethanol made from plant material then used as a fuel.
6) Methanol is safe to drink in small amounts because it is an alcohol like ethanol.